New Life Found That Lives Off Electricity
Last year, biophysicist Moh El-Naggar and his graduate student Yamini Jangir plunged beneath South Dakota’s Black Hills into an old gold mine that is now more famous as a home to a dark matter detector. Unlike most scientists who make pilgrimages to the Black Hills these days, El-Naggar and Jangir weren’t there to hunt for subatomic particles. They came in search of life.
In the darkness found a mile underground, the pair traversed the mine’s network of passages in search of a rusty metal pipe. They siphoned some of the pipe’s ancient water, directed it into a vessel, and inserted a variety of electrodes. They hoped the current would lure their prey, a little-studied microbe that can live off pure electricity.
The electricity-eating microbes that the researchers were hunting for belong to a larger class of organisms that scientists are only beginning to understand. They inhabit largely uncharted worlds: the bubbling cauldrons of deep sea vents; mineral-rich veins deep beneath the planet’s surface; ocean sediments just a few inches below the deep seafloor. The microbes represent a segment of life that has been largely ignored, in part because their strange habitats make them incredibly difficult to grow in the lab.
Yet early surveys suggest a potential microbial bounty. A recent sampling of microbes collected from the seafloor near Catalina Island, off the coast of Southern California, uncovered a surprising variety of microbes that consume or shed electrons by eating or breathing minerals or metals. El-Naggar’s team is still analyzing their gold mine data, but he says that their initial results echo the Catalina findings. Thus far, whenever scientists search for these electron eaters in the right locations — places that have lots of minerals but not a lot of oxygen — they find them.
As the tally of electron eaters grows, scientists are beginning to figure out just how they work. How does a microbe consume electrons out of a piece of metal, or deposit them back into the environment when it is finished with them? A study published last year revealed the way that one of these microbes catches and consumes its electrical prey. And not-yet-published work suggests that some metal eaters transport electrons directly across their membranes — a feat once thought impossible.
The Rock Eaters
Though eating electricity seems bizarre, the flow of current is central to life. All organisms require a source of electrons to make and store energy. They must also be able to shed electrons once their job is done. In describing this bare-bones view of life, Nobel Prize-winning physiologist Albert Szent-Györgyi once said, “Life is nothing but an electron looking for a place to rest.”
Humans and many other organisms get electrons from food and expel them with our breath. The microbes that El-Naggar and others are trying to grow belong to a group called lithoautotrophs, or rock eaters, which harvest energy from inorganic substances such as iron, sulfur or manganese. Under the right conditions, they can survive solely on electricity.
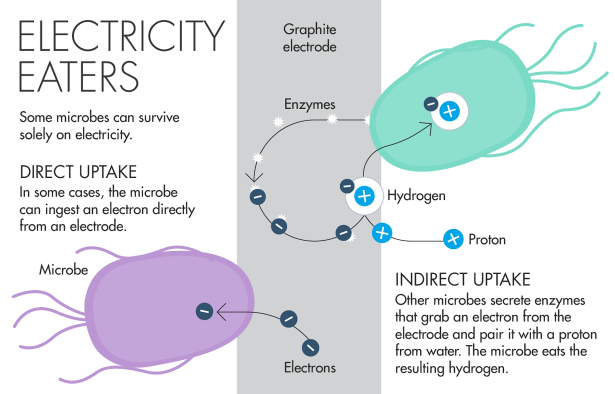
The microbes’ apparent ability to ingest electrons — known as direct electron transfer — is particularly intriguing because it seems to defy the basic rules of biophysics. The fatty membranes that enclose cells act as an insulator, creating an electrically neutral zone once thought impossible for an electron to cross. “No one wanted to believe that a bacterium would take an electron from inside of the cell and move it to the outside,” said Kenneth Nealson, a geobiologist at the University of Southern California, in a lecture to the Society for Applied Microbiology in London last year.
Lucy Reading-Ikkanda for Quanta Magazine
In the 1980s, Nealson and others discovered a surprising group of bacteria that can expel electrons directly onto solid minerals. It took until 2006 to discover the molecular mechanism behind this feat: A trio of specialized proteins sits in the cell membrane, forming a conductive bridge that transfers electrons to the outside of cell. (Scientists still debate whether the electrons traverse the entire distance of the membrane unescorted.)
Inspired by the electron-donators, scientists began to wonder whether microbes could also do the reverse and directly ingest electrons as a source of energy. Researchers focused their search on a group of microbes called methanogens, which are known for making methane. Most methanogens aren’t strict metal eaters. But in 2009, Bruce Logan, an environmental engineer at Pennsylvania State University, and collaborators showed for the first time that a methanogen could survive using only energy from an electrode. The researchers proposed that the microbes were directly sucking up electrons, perhaps via a molecular bridge similar to the ones the electron-producers use to shuttle electrons across the cell wall. But they lacked direct proof.
Then last year, Alfred Spormann, a microbiologist at Stanford University, and collaborators poked a hole in Logan’s theory. They uncovered a way that these organisms can survive on electrodes without eating naked electrons.
The microbe Spormann studied, Methanococcus maripaludis, excretes an enzyme that sits on the electrode’s surface. The enzyme pairs an electron from the electrode with a proton from water to create a hydrogen atom, which is a well-established food source among methanogens. “Rather than having a conductive pathway, they use an enzyme,” said Daniel Bond, a microbiologist at the University of Minnesota Twin Cities. “They don’t need to build a bridge out of conductive materials.”
Though the microbes aren’t eating naked electrons, the results are surprising in their own right. Most enzymes work best inside the cell and rapidly degrade outside. “What’s unique is how stable the enzymes are when they [gather on] the surface of the electrode,” Spormann said. Past experiments suggest these enzymes are active outside the cell for only a few hours, “but we showed they are active for six weeks.”
Spormann and others still believe that methanogens and other microbes can directly suck up electricity, however. “This is an alternative mechanism to direct electron transfer, it doesn’t mean direct electron transfer can’t exist,” said Largus Angenent, an environmental engineer at Cornell University, and president of the International Society for Microbial Electrochemistry and Technology. Spormann said his team has already found a microbe capable of taking in naked electrons. But they haven’t yet published the details.
Microbes on Mars
Only a tiny fraction — perhaps 2 percent — of all the planet’s microorganisms can be grown in the lab. Scientists hope that these new approaches — growing microbes on electrodes rather than in traditional culture systems — will provide a way to study many of the microbes that have been so far impossible to cultivate.
“Using electrodes as proxies for minerals has helped us open and expand this field,” said Annette Rowe, a postdoctoral researcher at USC working with El-Naggar. “Now we have a way to grow the bacteria and monitor their respiration and really have a look at their physiology.”
Rowe has already had some success.
In 2013, she went on a microbe prospecting trip to the iron-rich sediments that surround California’s Catalina Island. She identified at least 30 new varieties of electric microbes in a study published last year. “They are from very diverse groups of microbes that are quite common in marine systems,” Rowe said. Before her experiment, no one knew these microbes could take up electrons from an inorganic substrate, she said. “That’s something we weren’t expecting.”
Just as fishermen use different lures to attract different fish, Rowe set the electrodes to different voltages to draw out a rich diversity of microbes. She knew when she had a catch because the current changed — metal eaters generate a negative current, as the microbes suck electrons from the negative electrode.

Connie A. Walter and Matt Kapust
Yamini Jangir, then a graduate student in Moh El-Naggar’s lab at the University of Southern California, collects water from a pipe at the Sanford Underground Research Facility nearly a mile underground.
The different varieties of bacteria that Rowe collected thrive under different electrical conditions, suggesting they employ different strategies for eating electrons. “Each bacteria had a different energy level where electron uptake would happen,” Rowe said. “We think that is indicative of different pathways.”
Rowe is now searching new environments for additional microbes, focusing on fluids from a deep spring with low acidity. She’s also helping with El-Naggar’s gold mine expedition. “We are trying to understand how life works under these conditions,” said El-Naggar. “We now know that life goes far deeper than we thought, and there’s a lot more than we thought, but we don’t have a good idea for how they are surviving.”
El-Naggar emphasizes that the field is still in its infancy, likening the current state to the early days of neuroscience, when researchers poked at frogs with electrodes to make their muscles twitch. “It took a long time for the basic mechanistic stuff to come out,” he said. “It’s only been 30 years since we discovered that microbes can interact with solid surfaces.”
Given the bounty from these early experiments, it seems that scientists have only scratched the surface of the microbial diversity that thrives beneath the planet’s shallow exterior. The results could give clues to the origins of life on Earth and beyond. One theory for the emergence of life suggests it originated on mineral surfaces, which could have concentrated biological molecules and catalyzed reactions. New research could fill in one of the theory’s gaps — a mechanism for transporting electrons from mineral surfaces into cells.
Moreover, subsurface metal eaters may provide a blueprint for life on other worlds, where alien microbes might be hidden beneath the planet’s shallow exterior. “For me, one of the most exciting possibilities is finding life-forms that might survive in extreme environments like Mars,” said El-Naggar, whose gold mine experiment is funded by NASA’s Astrobiology Institute. Mars, for example, is iron-rich and has water flowing beneath its surface. “If you have a system that can pick up electrons from iron and have some water, then you have all the ingredients for a conceivable metabolism,” said El-Naggar. Perhaps a former mine a mile underneath South Dakota won’t be the most surprising place that researchers find electron-eating life.
