Jammed Cells Expose the Physics of Cancer

In 1995, while he was a graduate student at McGill University in Montreal, the biomedical scientist Peter Friedl saw something so startling it kept him awake for several nights. Coordinated groups of cancer cells he was growing in his adviser’s lab started moving through a network of fibers meant to mimic the spaces between cells in the human body.
For more than a century, scientists had known that individual cancer cells can metastasize, leaving a tumor and migrating through the bloodstream and lymph system to distant parts of the body. But no one had seen what Friedl had caught in his microscope: a phalanx of cancer cells moving as one. It was so new and strange that at first he had trouble getting it published. “It was rejected because the relevance [to metastasis] wasn’t clear,” he said. Friedl and his co-authors eventually published a short paper in the journal Cancer Research.
Two decades later, biologists have become increasingly convinced that mobile clusters of tumor cells, though rarer than individual circulating cells, are seeding many — perhaps most — of the deadly metastatic invasions that cause 90 percent of all cancer deaths. But it wasn’t until 2013 that Friedl, now at Radboud University in the Netherlands, really felt that he understood what he and his colleagues were seeing. Things finally fell into place for him when he read a paper by Jeffrey Fredberg, a professor of bioengineering and physiology at Harvard University, which proposed that cells could be “jammed” — packed together so tightly that they become a unit, like coffee beans stuck in a hopper.
Fredberg’s research focused on lung cells, but Friedl thought his own migrating cancer cells might also be jammed. “I realized we had exactly the same thing, in 3-D and in motion,” he said. “That got me very excited, because it was an available concept that we could directly put onto our finding.” He soon published one of the first papers applying the concept of jamming to experimental measurements of cancer cells.
Physicists have long provided doctors with tumor-fighting tools such as radiation and proton beams. But only recently has anyone seriously considered the notion that purely physical concepts might help us understand the basic biology of one of the world’s deadliest phenomena. In the past few years, physicists studying metastasis have generated surprisingly precise predictions of cell behavior. Though it’s early days, proponents are optimistic that phase transitions such as jamming will play an increasingly important role in the fight against cancer. “Certainly in the physics community there’s momentum,” Fredberg said. “If the physicists are on board with it, the biologists are going to have to. Cells obey the rules of physics — there’s no choice.”
The Jam Index
In the broadest sense, physical principles have been applied to cancer since long before physics existed as a discipline. The ancient Greek physician Hippocrates gave cancer its name when he referred to it as a “crab,” comparing the shape of a tumor and its surrounding veins to a carapace and legs.
But those solid tumors do not kill more than 8 million people annually. Once tumor cells strike out on their own and metastasize to new sites in the body, drugs and other therapies rarely do more than prolong a patient’s life for a few years.
Biologists often view cancer primarily as a genetic program gone wrong, with mutations and epigenetic changes producing cells that don’t behave the way they should: Genes associated with cell division and growth may be turned up, and genes for programmed cell death may be turned down. To a small but growing number of physicists, however, the shape-shifting and behavior changes in cancer cells evoke not an errant genetic program but a phase transition.
The phase transition — a change in a material’s internal organization between ordered and disordered states — is a bedrock concept in physics. Anyone who has watched ice melt or water boil has witnessed a phase transition. Physicists have also identified such transitions in magnets, crystals, flocking birds and even cells (and cellular components) placed in artificial environments.
But compared to a homogeneous material like water or a magnet — or even a collection of identical cells in a dish — cancer is a hot mess. Cancers vary widely depending on the individual and the organ they develop in. Even a single tumor comprises a mind-boggling jumble of cells with different shapes, sizes and protein compositions. Such complexities can make biologists wary of a general theoretical framework. But they don’t daunt physicists. “Biologists are more trained to look at complexity and differences,” said the physicist Krastan Blagoev, who directs a National Science Foundation program that funds work on theoretical physics in living systems. “Physicists try to look at what’s common and extract behaviors from the commonness.”
In a demonstration of this approach, the physicists Andrea Liu, now of the University of Pennsylvania, and Sidney Nagel of the University of Chicago published a brief commentary in Nature in 1998 about the process of jamming. They described familiar examples: traffic jams, piles of sand, and coffee beans stuck together in a grocery-store hopper. These are all individual items held together by an external force so that they resemble a solid. Liu and Nagel put forward the provocative suggestion that jamming could be a previously unrecognized phase transition, a notion that physicists, after more than a decade of debate, have now accepted.
Though not the first mention of jamming in the scientific literature, Liu and Nagel’s paper set off what Fredberg calls “a deluge” among physicists. (The paper has been cited more than 1,400 times.) Fredberg realized that cells in lung tissue, which he had spent much of his career studying, are closely packed in a similar way to coffee beans and sand. In 2009 he and colleagues published the first paper suggesting that jamming could hold cells in tissues in place, and that an unjamming transition could mobilize some of those cells, a possibility that could have implications for asthma and other diseases.
The paper appeared amid a growing recognition of the importance of mechanics, and not just genetics, in directing cell behavior, Fredberg said. “People had always thought that the mechanical implications were at the most downstream end of the causal cascade, and at the most upstream end are genetic and epigenetic factors,” he said. “Then people discovered that physical forces and mechanical events actually can be upstream of genetic events — that cells are very aware of their mechanical microenvironments.”
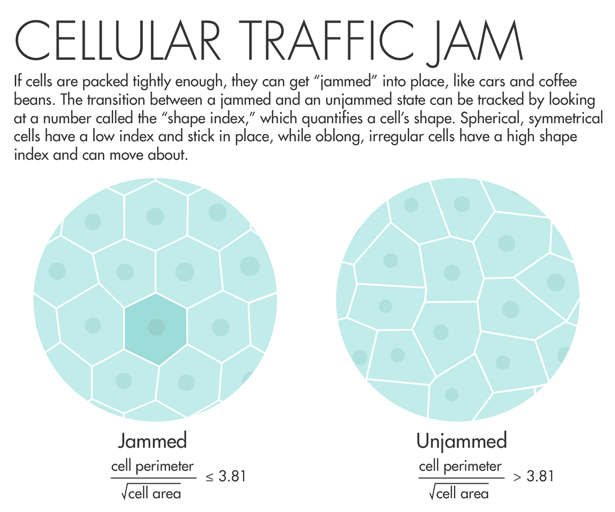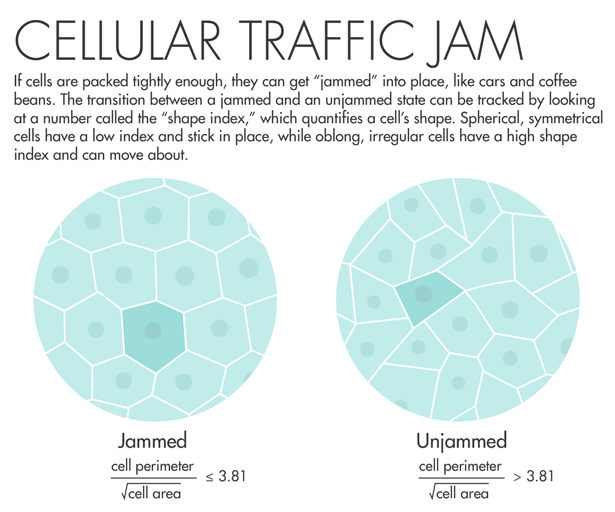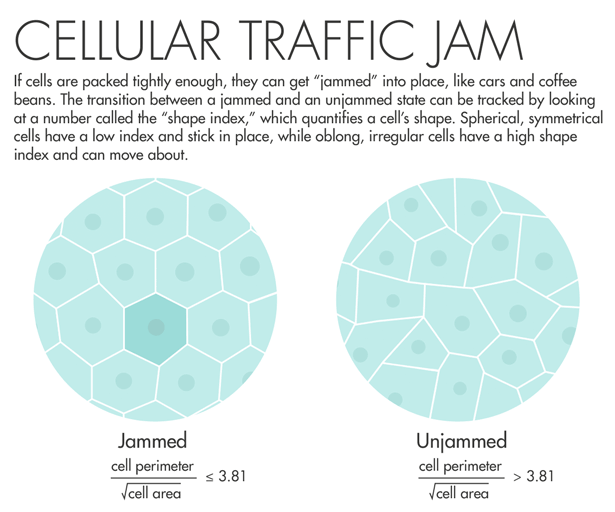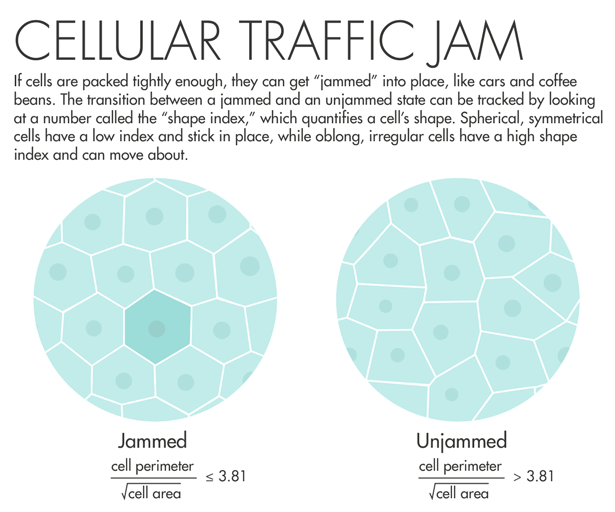
Lisa Manning, a physicist at Syracuse University, read Fredberg’s paper and decided to put his idea into action. She and colleagues used a two-dimensional model of cells that are connected along edges and at vertices, filling all space. The model yielded an order parameter — a measurable number that quantifies a material’s internal order — that they called the “shape index.” The shape index relates the perimeter of a two-dimensional slice of the cell and its total surface area. “We made what I would consider a ridiculously strict prediction: When that number is equal to 3.81 or below, the tissue is a solid, and when that number is above 3.81, that tissue is a fluid,” Manning said. “I asked Jeff Fredberg to go look at this, and he did, and it worked perfectly.”
Fredberg saw that lung cells with a shape index above 3.81 started to mobilize and squeeze past each other. Manning’s prediction “came out of pure theory, pure thought,” he said. “It’s really an astounding validation of a physical theory.” A program officer with the Physical Sciences in Oncology program at the National Cancer Institute learned about the results and encouraged Fredberg to do a similar analysis using cancer cells. The program has given him funding to look for signatures of jamming in breast-cancer cells.
Meanwhile, Josef Käs, a physicist at Leipzig University in Germany, wondered if jamming could help explain puzzling behavior in cancer cells. He knew from his own studies and those of others that breast and cervical tumors, while mostly stiff, also contain soft, mobile cells that stream into the surrounding environment. If an unjamming transition was fluidizing these cancer cells, Käs immediately envisioned a potential response: Perhaps an analysis of biopsies based on measurements of tumor cells’ state of jamming, rather than a nearly century-old visual inspection procedure, could determine whether a tumor is about to metastasize.
Käs is now using a laser-based tool to look for signatures of jamming in tumors, and he hopes to have results later this year. In a separate study that is just beginning, he is working with Manning and her colleagues at Syracuse to look for phase transitions not just in cancer cells themselves, but also in the matrix of fibers that surrounds tumors.
More speculatively, Käs thinks the idea could also yield new avenues for therapies that are gentler than the shock-and-awe approach clinicians typically use to subdue a tumor. “If you can jam a whole tumor, then you have a benign tumor — that I believe,” he said. “If you find something which basically jams cancer cells efficiently and buys you another 20 years, that might be better than very disruptive chemotherapies.” Yet Käs is quick to clarify that he is not sure how a clinician would induce jamming.
Castaway Cooperators
Beyond the clinic, jamming could help resolve a growing conceptual debate in cancer biology, proponents say. Oncologists have suspected for several decades that metastasis usually requires a transition between sticky epithelial cells, which make up the bulk of solid tumors, and thinner, more mobile mesenchymal cells that are often found circulating solo in cancer patients’ bloodstreams. As more and more studies deliver results showing activity similar to that of Friedl’s migrating cell clusters, however, researchers have begun to question whether go-it-alone mesenchymal cells, which Friedl calls “lonely riders,” could really be the main culprits behind the metastatic disease that kills millions.
Some believe jamming could help get oncology out of this conceptual jam. A phase transition between jammed and unjammed states could fluidize and mobilize tumor cells as a group, without requiring them to transform from one cell type to a drastically different one, Friedl said. This could allow metastasizing cells to cooperate with one another, potentially giving them an advantage in colonizing a new site.
The key to developing this idea is to allow for a range of intermediate cell states between two extremes. “In the past, theories for how cancer might behave mechanically have either been theories for solids or theories for fluids,” Manning said. “Now we need to take into account the fact that they’re right on the edge.”
Hints of intermediate states between epithelial and mesenchymal are also emerging from physics research not motivated by phase-transition concepts. Herbert Levine, a biophysicist at Rice University, and his late colleague Eshel Ben-Jacob of Tel Aviv University recently created a model of metastasis based on concepts borrowed from nonlinear dynamics. It predicts the existence of clusters of circulating cells that have traits of both epithelial and mesenchymal cells. Cancer biologists have never seen such transitional cell states, but some are now seeking them in lab studies. “We wouldn’t have thought about it” on our own, said Kenneth Pienta, a prostate cancer specialist at Johns Hopkins University. “We have been directly affected by theoretical physics.”
Biology’s Phase Transition
Models of cell jamming, while useful, remain imperfect. For example, Manning’s models have been confined to two dimensions until now, even though tumors are three-dimensional. Manning is currently working on a 3-D version of her model of cellular motility. So far it seems to predict a fluid-to-solid transition similar to that of the 2-D model, she said.
In addition, cells are not as simple as coffee beans. Cells in a tumor or tissue can change their own mechanical properties in often complex ways, using genetic programs and other feedback loops, and if jamming is to provide a solid conceptual foundation for aspects of cancer, it will need to account for this ability. “Cells are not passive,” said Valerie Weaver, the director of the Center for Bioengineering and Tissue Regeneration at the University of California, San Francisco. “Cells are responding.”
Weaver also said that the predictions made by jamming models resemble what biologists call extrusion, a process by which dead epithelial cells are squeezed out of crowded tissue — the disfunction of which has recently been implicated in certain types of cancer. Manning believes that cell jamming likely provides an overarching mechanical explanation for many of the cell behaviors involved in cancer, including extrusion.
Space-filling tissue models like the one Manning uses, which produce the jamming behavior, also have trouble accounting for all the details of how cells interact with their neighbors and with their environment, Levine said. He has taken a different approach, modeling some of the differences in the ways cells can react when they’re being crowded by other cells. “Jamming will take you some distance,” he said, adding, “I think we will get stuck if we just limit ourselves to thinking of these physics transitions.”
Manning acknowledges that jamming alone cannot describe everything going on in cancer, but at least in certain types of cancer, it may play an important role, she said. “The message we’re not trying to put out there is that mechanics is the only game in town,” she said. “In some instances we might do a better job than traditional biochemical markers [in determining whether a particular cancer is dangerous]; in some cases we might not. But for something like cancer we want to have all hands on deck.”
With this in mind, physicists have suggested other novel approaches to understanding cancer. A number of physicists, including Ricard Solé of Pompeu Fabra University in Barcelona, Jack Tuszynski of the University of Alberta, and Salvatore Torquato of Princeton University, have published theory papers suggesting ways that phase transitions could help explain aspects of cancer, and how experimentalists could test such predictions.
Others, however, feel that phase transitions may not be the right tool. Robert Austin, a biological physicist at Princeton University, cautions that phase transitions can be surprisingly complex. Even for a seemingly elementary case such as freezing water, physicists have yet to compute exactly when a transition will occur, he notes — and cancer is far more complicated than water.
And from a practical point of view, all the theory papers in the world won’t make a difference if physicists cannot get biologists and clinicians interested in their ideas. Jamming is a hot topic in physics, but most biologists have not yet heard of it, Fredberg said. The two communities can talk to each other at physics-and-cancer workshops during meetings hosted by the American Physical Society, the American Association for Cancer Research or the National Cancer Institute. But language and culture gaps remain. “I can come up with some phase diagrams, but in the end you have to translate it into a language which is relevant to oncologists,” Käs said.
Those gaps will narrow if jamming and phase transition theory continue to successfully explain what researchers see in cells and tissues, Fredberg said. “If there’s really increasing evidence that the way cells move collectively revolves around jamming, it’s just a matter of time until that works its way into the biological literature.”
And that, Friedl said, will give biologists a powerful new conceptual tool. “The challenge, but also the fascination, comes from identifying how living biology hijacks the physical principle and brings it to life and reinvents it using molecular strategies of cells.”
