Viruses Would Rather Jump to New Hosts Than Evolve With Them

When new species evolve, where do their viruses come from? As little more than free-ranging bundles of genetic material, viruses desperately need to hijack their hosts’ cellular machinery and resources to replicate, over and over again. Without its host, a virus is nothing.
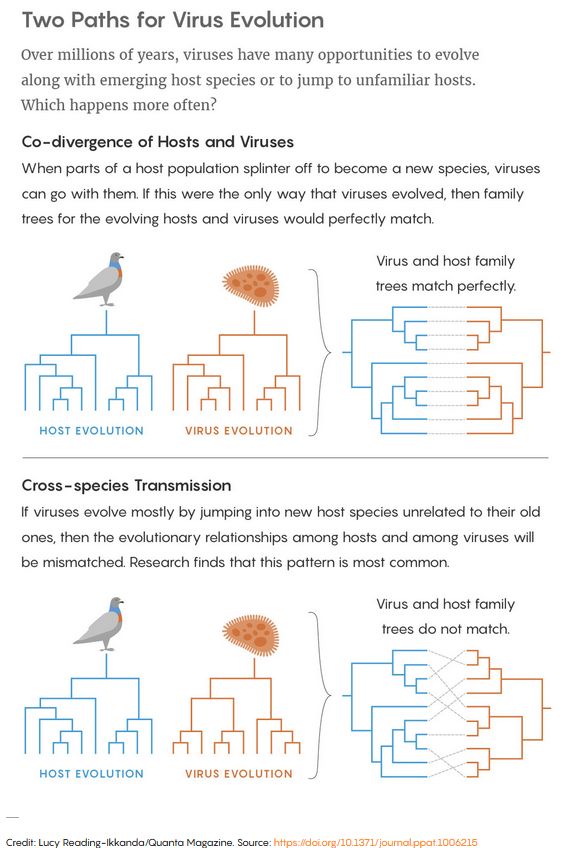
Because of that dependence, some viruses have stuck with their hosts throughout evolution, mutating to make minor adjustments every time the host branched into a new species — a process called co-divergence. Humans and chimpanzees, for instance, have slightly different versions of the hepatitis B virus, both of which likely mutated from a version that infected their shared ancestor more than four million years ago.
The other option — cross-species transmission — occurs when a virus jumps into a completely new type of host largely unrelated to its former one. That kind of viral evolution is notoriously linked to severe emerging diseases like bird flu, HIV, Ebola fever and SARS. Given the extreme virulence of those diseases, the apparent rarity of cross-species transmission seemed fortunate.
But recently, when researchers in Australia conducted the first study of the long-term evolution of thousands of diverse viruses, they reached a startling conclusion: cross-species transmission has been more important and more frequent than anyone realized. Jumps between species have driven most major evolutionary innovations in the viruses. Meanwhile, co-divergence has been less common than was assumed and has mostly caused incremental changes.
“They showed rather convincingly that co-divergence is the exception rather than the rule,” said Pleuni Pennings, an evolutionary biologist and assistant professor at San Francisco State University who was not involved with the study.
The finding does not necessarily mean that emerging diseases from cross-species transmission are a more grave or imminent threat than medical science has assumed. However, it does reveal that the dynamics of virus evolution can be surprisingly complex. If scientists have been underestimating how often viruses can move into new hosts, then understanding which viruses are most primed to do so becomes a higher priority.
There’s no shortage of reasons why cross-species jumps would seem unlikely to influence viral evolution much. The odds against a virus leaping successfully to a new host species are formidable. If the virus can’t manipulate the host’s genetic material and replicate itself, then that’s the end of the line. A virus might need to make multiple attempts to infect a novel host over decades or longer, accumulating appropriate mutations all the while, before it could finally establish itself, replicate and spread.
This past spring, for example, a team of naturalists and biomedical researchers led by Susan VandeWoude, professor of comparative medicine at Colorado State University, reported an example of what seems to be an incomplete cross-species transition. VandeWoude studies lentiviruses — a type of retrovirus that includes HIV — in mountain lions and bobcats. She and her team kept finding a certain bobcat lentivirus in mountain lions in California and Florida. But every time, genetic evidence showed the virus came from a mountain lion’s exposure to an infected bobcat — for instance, from eating one — and not from another infected mountain lion passing it on. The viral loads in the mountain lions were also low, meaning the virus was struggling to replicate.


Researchers recently found that mountain lions (left) in California and Florida sometimes carry a virus that is native to a different feline species, the bobcat (right). In all those cases, however, the mountain lion picked up the virus from exposure to an infected bobcat. Perhaps someday the virus will become infectious in the mountain lions, too, but for now, that cross-species transfer is incomplete. Nathan Rupert; Blake Matheson
In short, the virus was getting into the new cat hosts, but it wasn’t clicking with that body environment well enough to establish itself. “In multiple transmission events, there wasn’t any evidence that a new virus was now being replicative in the mountain lions,” VandeWoude said. (In contrast, VandeWoude’s team found that a form of the bobcat virus had made the jump into Florida panthers, which were passing around a variant adapted to them.) Given that the transmissions between the cat species are so frequent, the lentivirus may eventually mutate enough to find the mountain lions habitable, but so far that hasn’t happened despite many opportunities.
Moreover, when viruses do successfully leap from one species to another, they can become victims of their own success. Particularly in small, isolated populations — which are how many new species begin — highly virulent viruses can rapidly exhaust the supply of available hosts and burn themselves out.
It therefore seemed safe for virologists to assume that, even if cross-species jumps happen a lot over the long term, co-divergence of viruses and their hosts would represent the norm. But actual data supporting that position have been sparse. “The idea of perfect co-divergence is one of those things that you learn about, but then if you try to find good examples, they are really rare,” Pennings said.

Edward Holmes, professor of biology at the University of Sydney, and his research team discovered that transfers from one host species to another drive the most important evolutionary changes in most viruses. University of Sydney
Edward Holmes, professor of biology at the University of Sydney, and his Australian colleagues decided to confront this mystery head on. Using viral genome data, they reconstructed the evolutionary history of 19 major virus families, each of which contained between 23 and 142 viruses found in diverse hosts ranging from mammals to fish to plants. They created phylogenetic, or evolutionary, trees for both the virus families and their host species and then compared them. They reasoned that if a virus had largely co-diverged with its host, evolving right alongside it, then the virus’s phylogenetic tree should resemble its host’s: ancestral versions of the virus ought to have infected the host’s ancestors. But if the virus is jumping between species, the trees of the hosts and viruses will look different. How different depends on how many cross-species jumps are made.
Their study, published in PLOS Pathogens, reported that for all 19 virus families, cross-species transmissions were common. According to Holmes, he wasn’t surprised to see that every virus family they looked at had jumped between species, but he was surprised at the sheer number of times they’d done it in their histories. “They’re all doing it,” he said. “It’s quite extraordinary.”
As for why researchers hadn’t recognized the importance of cross-species jumps to virus evolution sooner, Holmes explained that previous phylogenetic studies had often taken too narrow a view, looking at relatively few species of hosts and viruses over short timescales. Over 10 or 20 years, you may not get a cross-species jump. “Over a million, you definitely will,” Holmes said.
Their novel approach “provides a framework to start looking at long-term associations between hosts and viruses,” said John Dennehy, an associate professor of biology at Queens College, about the study.
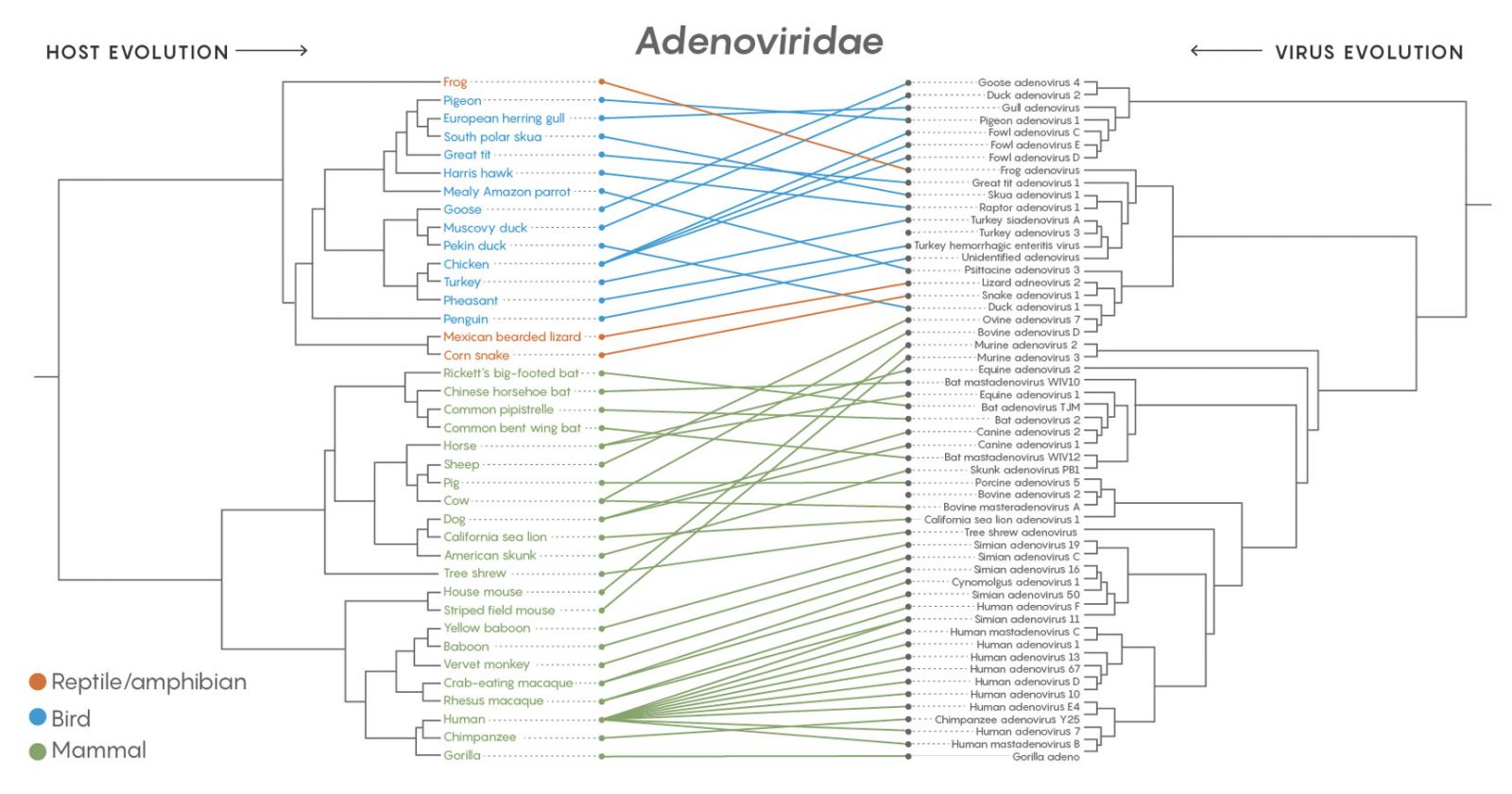
A comparison of the family tree of the adenoviruses (Adenoviridae) with the branching evolution of their host species shows that the viruses jumped to completely different hosts more than they co-diverged with emerging host species. The same was true of all 19 major families of virus examined in a long-term study. Credit: Lucy Reading-Ikkanda/Quanta Magazine. Source: https://doi.org/10.1371/journal.ppat.1006215
For Holmes and his colleagues, one insight into how and why cross-species transmission happens came from their observation that RNA viruses (which use RNA as a genetic material) seem to jump species much more frequently than DNA viruses (which use DNA) do. “That’s probably because they have a higher mutation rate,” VandeWoude said. For RNA viruses, the combination of a generally smaller genome and a higher mutation rate makes it more likely that they can adapt to a new host environment.
Holmes pins the trend to the different life histories of RNA and DNA viruses, too. RNA virus infections are often acute but transient, coming and going in a relatively short time — as with the flu or the common cold. That transience means the virus can miss its opportunity to be part of the diverging host species. “If you’re an acute virus, you only have an effect for a few days or weeks,” Holmes said. “Co-divergence is very hard to do on average. You’re not around long enough.”
In contrast, DNA virus infections are often chronic. When part of a host population splinters off to become a new species, it’s more likely to bring the virus along because more of the population would be infected. That increases the chances the virus can co-diverge with its new hosts.

Bats roost and hibernate together in gigantic numbers, which increases the opportunities for them to pass around novel viruses they have acquired. Craig Stihler, West Virginia Division of Natural Resources
The host’s way of life also plays a role in virus transmission and the odds of co-divergence versus cross-species jumping. “We know that host population size and density are really important in dictating how many viruses they have,” Holmes said. He used bats as an example: Bats have a penchant for carrying many different viruses, but that’s at least partly because “there are a hell of a lot of bats.” With such large populations, they’re just more likely to contract viruses. “A very simple rule of ecology is, the more hosts there are, the more virulent things they can carry,” Holmes said. “The chance of a virus finding a susceptible host is just higher.”
A 1975 Science paper from Francis L. Black of Yale University provided some insight into how human diseases are affected by a host’s population dynamics. Looking at fairly isolated, small societies of native Amazonian people, researchers found that although they could often detect chronic viral infections, acute infections were largely absent. Isolation kept the tribes protected from new viruses. The few viruses that did get in, if they were acute, quickly worked their way through the small tribes and died out. Without lots of hosts to sustain them, the viruses disappeared quickly.
The Risk of New Diseases
The discovery that cross-species transmission has occurred so often might seem worrisome, given its association with harsh emerging diseases. With so many jumps having occurred in the past, does the future hold more of the same?
Not necessarily. “Historical jumping rates do not necessarily predict the future, especially when it comes to humans,” Pennings said. The way we live today is so different from how humans lived just a few centuries ago, our risk of emerging diseases is probably different as well.

The Kayapó people were among the relatively isolated indigenous Amazonian societies studied in a classic 1975 paper on population dynamics and viral diseases. Scientists observed that the Kayapó suffered mostly from chronic viral infections, probably because isolation and the limited number of hosts discouraged acute ones .Tatiana Reis
Humans carry a lot of viruses as well. We too have large populations, and we’re incredibly mobile, meaning we bring along viruses to new, susceptible hosts quite easily. “We have all kinds of behaviors that put us at risk for all kinds of things because we like to go poke around in places where we probably shouldn’t, we take lots of risks, we eat things we probably shouldn’t eat,” VandeWoude said. “We’re probably the worst offenders and probably the biggest target for cross-species transmission just because we do so many crazy things.”
And doing those crazy things often leads us to bump against other species. The more we do that, the more we expose ourselves to new viruses, and the species putting us at greatest risk are the ones we interact with the most. “We’re more likely to get something from rats than from tigers,” Pennings said.
But further studies of viral evolutionary history may help scientists figure out whether there are species to which we should pay more attention as sources of new infections. (Epidemiologists already closely monitor viruses at risk for passing from poultry to humans because of fears about bird flu.) Maybe viruses from plants, fish and mammals are equally risky to humans, or maybe those researchers trying to predict the next epidemic can narrow their focus to a select high-risk group.
Holmes has a different view. “I don’t think prediction is in any way viable at all,” he said. “I understand why it’s done, but what I think I get from this and the sheer number of new viruses we’re discovering is it’s just not viable.”
Luckily, it’s now much easier to do that sort of analysis with the growth of metagenomics, the study of genomic information extracted from the environment. For this study, Holmes and his colleagues pulled viral genomic sequences from a number of publicly accessible databases. They didn’t need to have physical samples of any of the viruses, which is a relatively new direction for this field. “Virology is moving into a new phase where, with metagenomics, you can now do this mass sampling stuff just to see what’s there,” Holmes said.
Holmes also noted that because it’s now so easy to access new information about viruses, the phylogenetic trees that he and his colleagues created will change a lot in the near future. “In three years’ time, these trees will be much fuller because we’ll have found so many new samples of these viruses,” he said.
_________________________________________________________
Mallory Locklear is a freelance science and tech writer with a PhD in neuroscience. She's based in New York and has bylines in Motherboard, New Scientist, Discover Magazine and more.
