The Political Economy of COVID-19 Vaccines

The COVID-19 pandemic has been unusual in several ways: the disproportionate extent to which people in rich countries (particularly in Europe and North America) have been affected; the sheer scale of the policy response for containment; and the speed and urgency of the global response.
The active interest in controlling the pandemic in rich countries shaped individual national responses as well as global policy. There was a massive push for vaccine development, through large subsidies for research and development to drug companies, pre-orders of vaccines, and other support by the US, Russia, China, and European countries.
This led to the rapid development of multiple COVID-19 vaccine candidates and even more rapid regulatory approval to several of them. Typically, vaccines take several years to be developed and approved, partly because of extended clinical trials to check for all possible responses. But some COVID-19 vaccine candidates were given official approval in Russia and China even before the essential Phase III trials were completed. Even in the U.S. and Europe, regulatory processes were accelerated, sweeping aside the usual demands for complete data and without checking for possible side effects.
Despite such proactive policy, the production and distribution of COVID-19 vaccines has exposed and intensified global inequality. Three features stand out: blatant vaccine grab by rich countries; protection of patent rights by governments in advanced countries, which prevents wider production of vaccines; and the use of vaccine distribution to promote both nationalism and diplomatic ‘soft power’.
The great vaccine grab
It seems obvious that a pandemic can be overcome only when it is overcome everywhere. The delayed vaccination of people across the world increases the possibility of virus mutation, reducing the ability to control the pandemic even in rich countries that have bagged vaccines. Prolonged fear of infection, because of inadequate vaccination, affects economic prospects, inhibiting and delaying global economic recovery. These risks are so great that rich countries would still benefit even if they decided to pay on their own for vaccinating all of the world’s population.
An ‘every-country-for-itself’ approach is irrational and even counterproductive. Yet that is exactly what has happened.
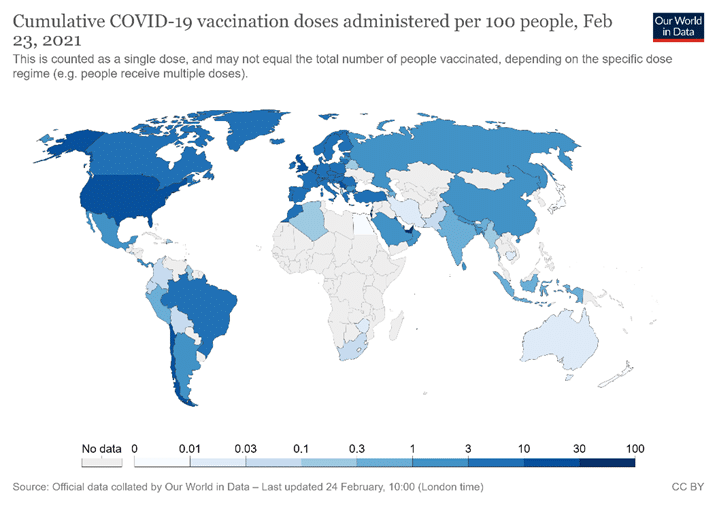
When three major vaccine candidates (from Pfizer-BioNTech, Moderna, and AstraZeneca) were approved in the U.S. and Europe, rich countries scrambled to lay claim to vaccine doses, confirming that wealthy countries and individuals would monopolise early doses of any effective vaccine. As a result, by late February 2021 COVID-19 vaccinations were heavily concentrated in the developed world (Figure 1).
Figure 1. Source: Coronavirus (Covid-19) Vaccinations. Accessed on 24 February 2021
This need not have occurred. The COVID-19 Vaccines Global Access Facility (COVAX) led by the World Health Organisation (WHO), the Coalition for Epidemic Preparedness Innovations, and Gavi, was established precisely to prevent this outcome, to prevent hoarding by rich countries and ensure access for the world’s poor. COVAX aims to accelerate COVID-19 vaccine development, secure doses for all countries, and distribute those doses fairly, beginning with the highest-risk groups. By early 2021, 190 countries, representing most of the world’s population, had joined. In February, the United States (which had been kept out by former president Donald Trump) also joined. Higher-income and middle-income countries will have access to the vaccines in the COVAX list and pay for their doses individually. The 92 lower-income member countries are to receive their doses free of charge.
The COVAX plan is to distribute vaccines in two phases. In the first phase, all participating countries would receive doses proportionate to their populations, beginning with enough doses to immunise the 3% of their population at highest risk, especially frontline workers in health and social care. Additional doses would then be delivered to cover 20% of each country’s population, beginning with others most in danger, such as the elderly and those with co-morbidities. In the second phase, vaccines would be delivered to specific countries based on how quickly the virus is spreading; whether other pathogens (like measles) are also spreading; and how vulnerable the country’s health infrastructure is to being overwhelmed. Eventually, everyone would be covered.
This is a fair system, given constraints on production. But the facility still remains underfunded, thus far raising only $4 billion of its modest target of $6.8 billion for 2021. Even worse, it has not been able to purchase the vaccines required for free distribution to poor countries as much as planned. This is because the COVAX facility allows member countries to make their own separate purchases directly from pharma companies. As a result, rich countries have competed to secure bilateral deals with pharmaceutical companies outside of COVAX. Within a month of the regulatory approval being granted to the first three vaccines, advanced countries, accounting for only 14% of the world’s population, had placed orders for around 85% of the estimated entire production for 2021 (Ghosh 2020).
Much of this was in the form of pre-orders even before regulatory approval was granted. Thirteen of the 48 firms engaged in COVID-19 vaccine development had made advance sales by mid-November 2020, promising to deliver 7.5 billion doses of vaccines, mostly to advanced countries, even before emergency-use authorisation had been granted. In some cases, this happened even before the clinical trials necessary for regulatory approval had been completed (Acharya and Reddy 2021).
Forty-four bilateral deals between governments and pharmaceutical companies (dominated by rich countries) were signed last year, and at least 12 have already been signed this year. Canada has ordered vaccines that could provide for more than 10 times its population–and then sought to get vaccines from COVAX as well. The U.S. has ordered vaccines equivalent to more than four times its population. Rich countries are now stockpiling vaccines that they have grabbed but are unable to distribute. Firms preferred to sell in these bilateral deals because they could charge higher prices than offered by COVAX. They typically keep secret the basic elements of the deal, including the price at which the vaccines were being provided to these governments.
This vaccine grab by rich countries meant that most of the world would get safe and approved vaccines only in 2022, and in some cases not even until 2024. In mid-January 2021, the head of the WHO noted that while 39 million vaccine doses had already been administered in the rich countries, in one poor country only 25 doses (in total) had been given, and 170 poorest countries had received no vaccines at all. He said: “I need to be blunt: the world is on the brink of a catastrophic moral failure–and the price of this failure will be paid with lives and livelihoods in the world’s poorest countries” (UN News, January 2021).
The unjustified protection of intellectual property
Insufficient production is an important reason for the poor and unequal distribution. Yet this scarcity is completely unnecessary and could be easily and rapidly remedied. The major factor limiting supply of approved vaccines is the persistence of patent rights that give pharmaceutical companies a monopoly on production, confining supplies to their own capacities and the few production licences they choose to issue to others.
Patents are usually seen as providing a necessary financial reward for invention/innovation, without which technological change would either not occur or be more limited. Big pharma (which has been the major lobby pushing for inclusion of intellectual property rights in the World Trade Organization (WTO) and in subsequent trade and economic partnership agreements) argues that developing new drugs requires such incentives because the costs are very high and drugs may not succeed even after years of effort.
Yet for COVID-19 vaccines, many big pharma companies received massive subsidies from governments that have mostly and in some cases completely covered research and development costs. In the U.S. alone, the six major vaccine companies received over $12 billion in public subsidies for developing COVID-19 vaccines (MSF 2021). Other rich country governments have provided similar subsidies. Private pharma companies also benefited from prior public research (Scientific American 2020) and reduced costs of clinical testing, because of more unpaid volunteers for trials. The ‘leader’ vaccines may have already received what could be considered as reasonable returns on their own investment, and more. For example, while Pfizer did not receive direct subsidies from the U.S. government, I received pre-orders for 100 million doses for $1.95 billion (Industry Week 2020). Moreover, it relied on technology from BioNTech, which had received $445 million from the German government for their research (Bloomberg 2020). Pfizer claims costs of $3.1 billion to develop this vaccine (BBC 2020), while estimated sales in 2021 will be worth $15 billion (Quartz, 2020). Developing the Moderna vaccine cost $2.5 billion, apparently entirely funded by the U.S. federal government (USA Today, 2020). The recently approved Johnson and Johnson vaccine benefited from U.S. government subsidies and a pre-order of 100 million doses likely to cover costs (Johnson and Johnson 2020).
The case of the AstraZeneca vaccine is particularly instructive, also because it is seen as viable for developing country use. (Significant quantities of this vaccine are being produced by the Serum Institute of India under a collaboration agreement.) The vaccine was entirely developed by a publicly funded lab at Oxford University. The original distribution model was for an open-licence platform, designed to make the vaccine freely available for any manufacturer. However, the Gates Foundation, which had clout because it had donated $750 million to Oxford for vaccine development, persuaded the university to change course completely and sign “an exclusive vaccine deal with AstraZeneca that gave the pharmaceutical giant sole rights and no guarantee of low prices” (Jay Hancock 2020).
Oxford and AstraZeneca promised not to make profits from sale of the vaccine, but the details were left vague. While Oxford will receive no royalties during the pandemic, it could subsequently gain from patents including those held by Vaccitech, a for-profit spinoff.
Meanwhile, AstraZeneca is charging differential prices for its vaccines sent to different countries, with some poorer countries paying higher rates. The European Union pays $3.50 per dose, while Bangladesh pays $4, and South Africa as much as $5.25 (Politico, 2021). (The more expensive vaccines are being provided by the Serum Institute of India.)
This variation in prices is not confined to the AstraZeneca shot. Because of competition for doses and opacity in contracts, the range of reported prices of vaccines is vast: from $2.19 to as much as $44 per dose, as of 1 March. (UNICEF, Covid Dashboard).
This restricted production creating unseemly vaccine grabs, overpriced and differentially priced doses determined by private suppliers, and inadequate provision for most of the world’s population, could all have been avoided if a proposal brought by India and South Africa to the WTO in October 2020 had been accepted. The proposal was for a waiver of obligations to enforce patents and other intellectual property rights related to COVID-19 products (MSF, November 2020).
This would mean that WTO members could choose not to grant or enforce patents and other intellectual property related to all COVID-19 drugs, vaccines, diagnostics, and other technologies, including masks and ventilators, for the duration of the pandemic. They could also more easily collaborate in research and development, technology transfer, manufacturing, scaling up, and supplying COVID-19 tools.
Most developing countries have supported this, but advanced countries have repeatedly blocked it in the TRIPS council of the WTO (Prabhala et al 2020). This is surprising, because such suspension would also benefit populations in the advanced countries by making available more vaccines quickly. A larger supply would reduce costs of additional vaccines, making them cheaper for governments and taxpayers across the world.
The blocking of the proposal at the WTO is presumably because of the lobbying power of multinational pharma companies, which have thus far been successful in preventing the TRIPS council from approving this on five separate attempts. (Incidentally, Bill Gates has refused to back this proposal (Mail & Guardian 2021).
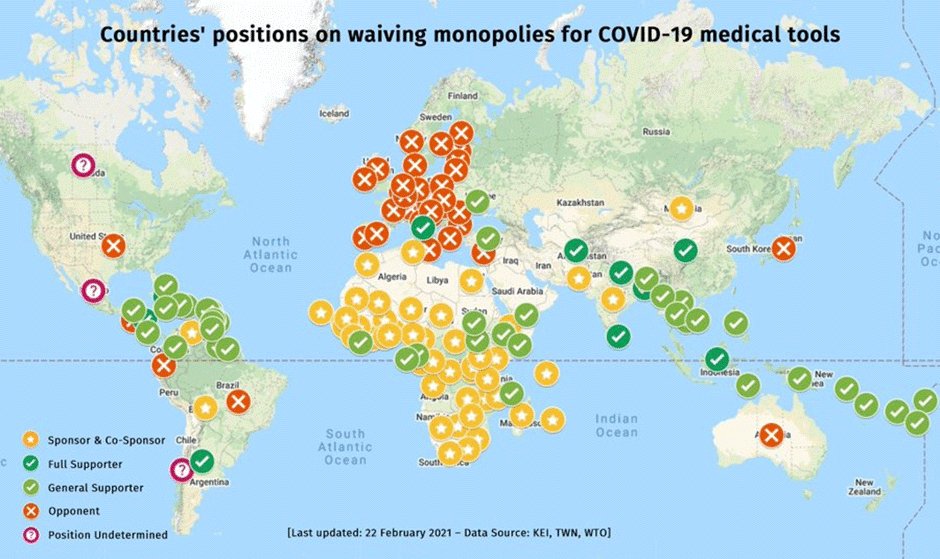
Figure 2 indicates how responses to this proposal in the WTO have closely tracked the persistent divide between global North and South. Rich countries that are home to the major multinational pharma companies have blocked it. These countries have already secured more than their requirements of COVID-19 vaccines. (It is a different matter that most of them have been less successful in distributing them quickly within their own countries, so they are now stockpiling vaccines.)
Figure 2. Source: Medecins san Frontiers, accessed 21 February 2021
Some have argued that this proposal is not necessary, since the WTO agreement on TRIPS already allows for compulsory licensing. The 2001 Doha Declaration on TRIPS and Public Health explicitly mentions public health emergencies as adequate cause to issue compulsory licences. A compulsory licence is an authorisation granted by a government to a third party to produce a patented product or process, without the express consent of the patentee (See WTO FAQs). It allows a government to override the patentee’s exclusive right to keep others from using its patented inventions. The idea is to prevent monopolistic behaviour, like preventing others from producing and charging excessively high prices. Conditions for compulsory licencing are obviously met in this pandemic, which is clearly a public health emergency. Some countries, like Chile and Israel, have already passed resolutions for such licences to be issued in the wake of the pandemic.
However, the difficulty with issuing compulsory licences in individual developing countries is that the transfer of technology by the inventor to other licenced producers is not compulsory. For pharmaceuticals, if the chemical composition of the product is known, the product can be reverse engineered and produced by other companies. When the precise technology for producing the vaccine is not known, compulsory licencing works only when patent holders are willing to make available the technology to licenced producers. In the case of COVID-19 vaccines, the big pharma companies are happy to supply rich countries that are already competing for privileged access to the limited vaccine supply, and therefore are not really concerned about access to smaller or less well-endowed markets. A global waiver would change those incentives for companies.
Therefore, a global move for suspension and/or modification of intellectual property rights for matters relating to essential public health concerns is essential. Since there is as yet no information on the immunity period offered by most of the vaccines, the suspension might be required for a more extended period. Such exemptions would be required not just for vaccines but for other treatments, tests, and products related to the pandemic, which may be required for the next few years.
Another idea is that of “voluntary pooling”, proposed by Costa Rica and supported by the WHO, which has created the COVID-19 Technology Access Pool (CTAP). This creates a pool of rights to tests, medicines and vaccines, with free access or licensing on reasonable and affordable terms for all countries. But so far only 40 (developing) countries have joined, and the major players have kept away. Lack of international support has meant that CTAP is not really effective thus far–but it may become significant in future, extending beyond the current COVID-19 pandemic to health emergencies in the future.
Regulatory approval and public trust
Other vaccine candidates being developed elsewhere also have the potential to combat the pandemic and ease the current shortages. The Sputnik V vaccine developed in Russia and the Sinovac and Sinopharm vaccines developed in China are reportedly effective. There are other vaccines being developed in India, Cuba, and elsewhere. Some have concerns about inadequate testing and hasty regulatory approval without the required trials and other processes. But even when these vaccine candidates are found to be safe and effective through clinical trials, there are further hurdles to their being accepted internationally.
This is largely because the WHO’s approval process is heavily skewed in favour of vaccines developed in the rich countries. The WHO has a list of ‘stringent regulatory authorities’ it trusts for quality control, which are only from developed countries in Europe, the US, Canada, Australia, and Japan. For the rest of the world, vaccine (and other drug) candidates are required to go through ‘prequalification’— a much more complicated and extended process. This greatly prolongs the time taken before vaccines from other countries are approved.
For example, the WHO approved the Pfizer-BioNTech vaccine at the end of 2020, less than two months after application, because WHO collaborates with the European Medicines Agency (EMA). However, the Russian (Sputnik) and Chinese (Sinovac and Sinopharm) vaccines, which had applied for approval even before the Pfizer-BioNTech vaccine, have still not received the WHO approval (Prabhala and Ling, 2021). All three of these companies can each produce up to 1 billion doses of vaccine in 2021 and have licensed production to other producers in developing countries.
In this pandemic, the usual regulatory standards have been greatly relaxed across the world, including in developed countries. No COVID-19 vaccine has been developed or released as transparently as it should have been. Even if WHO feels the regulatory standards in other countries might be less stringent, it could still work with different national regulatory authorities to ensure that all vaccine candidates are treated on an equal footing. Given the global scarcity that is denying people in poor countries access to vaccines, the WHO should take a proactive approach to enable global distribution of such vaccines when they meet some harmonised standards.
If this is done, it may be possible to circumvent the stranglehold of the big pharma companies on COVID-19 vaccines, which enables private profiteering in the midst of a health crisis and widespread economic distress. Some countries have already approved these other vaccine candidates for domestic use and have benefited from this access.
For example, by late February 2021 Chile had managed to provided vaccine doses to 17% of its population, by relying on imports of the China’s Sinovac, which is also being used in Bolivia, Brazil, Indonesia, and Turkey. Several countries have approved the Sinopharm vaccine, including UAE, Bahrain, Egypt, Jordan, Iraq, Serbia, Morocco, Hungary, and Pakistan. The Sputnik V vaccine is likely to be produced and distributed in several countries across Europe, the Middle East, Africa, and Latin America.
India should have been a prime example of successful production and distribution of COVID-19 vaccines. It has a number of major vaccine companies, has a long and successful history of inoculation drives, and until recently, there was a high degree of public trust in vaccines. Unfortunately, over-enthusiastic attempts by the government to first promote and then push particular vaccine candidates (Covaxin produced by Bharat Biotech) even before the required clinical trials were completed may have proved to be counterproductive, reducing public trust within and outside India. There have been many reports of people in India–including health workers–refusing to be vaccinated with Covaxin.
This is unfortunate, since once such trust has been lost, it takes time to be remedied and reversed. It also has an impact on India’s ability to export vaccines now and in the future. A recent YouGov poll of 19,000 people in 17 developed countries found that vaccines developed in Russia, China, and India ranked lowest in popular perceptions of efficacy, just above Iran which has very little vaccine production (Prabhala and Ling, 2021). India has also been plagued by major issues in distribution, belying expectations and past experience of vaccination drives.
To be fair, most countries have shown poor performance in vaccine rollout, including the developed countries that have sought to grab many multiples of their required shares of global supply. Overall, domestic distribution of vaccines has mostly mirrored the global distribution: unequal, unjust, and incompetent. This approach will delay the resolution of the ongoing pandemic and creates concerns about humanity’s ability to co-operate to address with the even greater challenges ahead.
Jayati Ghosh is professor of economics at Jawaharlal Nehru University, New Delhi, and the executive secretary of International Development Economics Associates (IDEAS). She is closely involved with a range of progressive organisations and social movements. She has written for Monthly Review and blogs at triplecrisis.com and networkideas.org/jayati-blog.
This article was originally published: The Indian Forum (March 5, 2021)