How RFK Jr. Broke 60 Years of Vaccine Decision-Making Process

Yesterday, in a move that stunned the public health community, HHS Secretary Robert F. Kennedy Jr. announced via Twitter/X that COVID-19 vaccines have been removed from the CDC’s recommended immunization schedule for healthy children and pregnant women.
This announcement is unprecedented. It bypassed every established scientific and administrative process typically involved in changing national vaccine policy. There was no consultation with the CDC’s Advisory Committee on Immunization Practices (ACIP). No formal recommendations were issued. No public health agencies released supporting documentation. As of Tuesday morning, the CDC’s website still reflected previous guidance recommending COVID-19 vaccination during pregnancy and for children aged 6 months and older.
And yet, with one unilateral, unprecedented, and unaccountable post, the Secretary of HHS overrode decades of rigorous vaccine decision-making processes and likely dismantled vaccine access for millions.
Worse yet, without any transparency into the decision-making process, this move takes away people’s freedom to choose an effective tool for protection against a dangerous disease. Let’s discuss…
What Was Announced
Secretary RFK Jr. was joined by NIH Director Jay Bhattacharya, who called the move "common sense and good science," and FDA Commissioner Marty Makary, who stated, "There is no evidence healthy kids need it today."
Let's be clear: Determining which groups continue to benefit most from COVID-19 vaccines is a legitimate scientific question that deserves careful study.
That’s why the FDA’s advisory committee (VRBPAC) discussed this just last week, and why the ACIP is scheduled to review it next month. These committees were already examining whether to narrow COVID-19 vaccine recommendations through the proper evidence-based process, making this unilateral announcement all the more troubling. However, good science answers a question like this by rigorously evaluating evidence and reaching conclusions based on data, not ideology.
While the benefits of COVID-19 vaccination for healthy children may be modest, pregnant women face elevated risks from COVID-19, and vaccination provides protection for both mother and baby. COVID-19 continues to cause hundreds of deaths weekly in the US, underscoring that this remains a significant health threat, warranting careful consideration of vaccine policy changes.
In fact, the same week that Makary and his colleague Vinay Prasad published a perspective piece in The New England Journal of Medicine calling for a more targeted approach to COVID-19 vaccination, they explicitly listed pregnancy as a condition warranting COVID-19 vaccination. No explanation has been given for the abrupt reversal.
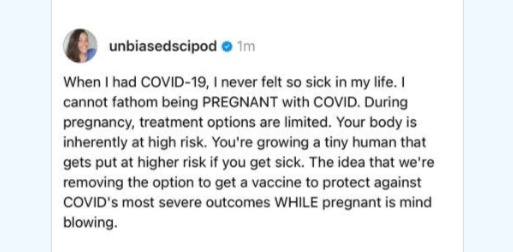
A post shared by @unbiasedscipod
What This Means
The practical implications of this announcement are profound, even if the legal authority behind it remains unclear:
-
Insurance coverage is at risk: Under the ACA, insurers must cover vaccines recommended by the CDC without cost-sharing. If the COVID-19 vaccine is removed from the schedule, insurers (including Medicare and Medicaid) may stop covering it for low-risk groups.
-
Providers may stop offering it: Most health systems, pharmacies, and providers follow CDC and ACIP guidance. If a vaccine is no longer recommended, it may no longer be stocked or administered.
-
The Vaccines for Children (VFC) program could be affected: This program only covers vaccines included on the CDC schedule. Millions of underserved children could lose access.
-
Manufacturer supply may decline: If demand collapses due to the policy change, vaccine manufacturers may stop producing pediatric and pregnancy-specific formulations.
-
Patients lose the freedom to choose: This move does not ban the vaccine, but removing it from the schedule likely makes it functionally inaccessible to many who still want it.
-
Legal challenges are likely: Policy experts have noted this decision could be challenged in court due to a lack of administrative process and failure to follow established protocols.
Why This Process Matters
For more than 60 years, the U.S. vaccine recommendation process has involved careful deliberation by independent scientists and public health leaders. The ACIP—a diverse panel of independent experts representing medicine, public health, health economics, ethics, and the public—reviews evidence and invites public comment before making any recommendation.
This decision bypassed all of that.
Even if the ultimate policy conclusion might have been similar, the process matters. We have these deliberative structures precisely because vaccine policy affects millions and requires careful, transparent evaluation of complex trade-offs. As of this writing, no official policy documents have been released. No clear definitions have been provided for who qualifies as "healthy" versus "high-risk." Pregnant women with underlying conditions were not even mentioned in the announcement.
This type of unilateral decision-making undermines public trust, erodes the credibility of our health institutions, and politicizes what should be an evidence-based process.
What Other Countries Are Doing
Globally, many countries are reassessing their COVID-19 vaccine policies, particularly for younger and lower-risk groups. But even in places where universal recommendations have been paused, vaccines typically remain available, and policy changes follow established advisory processes.
-
The UK removed COVID-19 vaccine recommendations for pregnancy based on formal cost-effectiveness analysis through their established advisory process (JCVI). The UK never had universal recommendations for healthy children, maintaining a cautious approach since the pandemic began.
-
Most European countries maintain "permissive" policies—vaccines remain available but aren't universally recommended for healthy children and during pregnancy. Some countries explicitly state that pregnancy is a high-risk category that warrants vaccination.
-
PAHO (Pan American Health Organization) still lists pregnant people as a high-priority group.
Key difference: Unlike the US announcement, international policy changes have followed standard advisory processes with formal cost-effectiveness analyses and transparent deliberation.
What About Other US Organizations?
-
ACOG (American College of Obstetricians and Gynecologists) continues to recommend COVID-19 vaccination during pregnancy. In a statement released yesterday, ACOG president Dr. Steven Fleischman emphasized how dangerous COVID-19 infection can be during pregnancy, and that “despite the change in recommendations from HHS, the science has not changed. It is very clear that COVID-19 infection during pregnancy can be catastrophic and lead to major disability, and it can cause devastating consequences for families. The COVID-19 vaccine is safe during pregnancy, and vaccination can protect our patients and their infants after birth.”
-
After the NEJM article dropped (and before yesterday’s bombshell), the AAP (American Academy of Pediatrics) expressed serious concerns that children may not have access to COVID-19 vaccines this fall unless they have underlying health conditions. With today’s announcement, that is a certainty. AAP leaders stated today that they fear that this decision will “leave children at risk”.
-
The Infectious Disease Society of America (IDSA) released the following statement: “New COVID vaccine recommendations threaten access, undermine choice, and will negatively impact health…IDSA strongly urges insurers to maintain coverage for COVID-19 vaccines so that all Americans can make the best decisions to protect themselves and their families against severe illness, hospitalization, and death.”
Bottom Line
This is not just about one vaccine. It’s about how we make public health decisions.
Good faith experts can disagree about the optimal COVID-19 vaccine policy for different groups. But those disagreements should be hashed out through established channels with independent advisors, public input, and transparent deliberation—not decided by executive fiat on social media.
Unless this action is reversed through legal or regulatory channels, it will likely mean that healthy children and pregnant individuals who want COVID-19 vaccination will no longer be able to access it. That’s a decision to restrict choices that should not be taken lightly and should follow the established rigorous scientific processes for making such a complex and important decision.
It’s not evidence-based. It’s not transparent. And it’s not how public health should work.
We should all be deeply concerned when a single official can override decades of process with a tweet.
Stay Curious,
Unbiased Science
P.S. Want to support our work? The best way is to subscribe to our Substack and share our content. While all our articles are always completely free to read, paid subscriptions help sustain our in-depth reporting on public health and science topics. Thank you for considering it!
Dr. Jessica Steier is a public health scientist with expertise in public health policy, biostatistics, and advanced analytics.
David Higgins, MD, MPH, is a physician, researcher, writer, and speaker standing at the intersection of pediatrics, preventive medicine, and public health. Subscribe to David
Elana Pearl BenJoseph, MD, MPH, is a physician with focus on pediatrics, health communication, and public health. Subscribe to Elana
Aimee Pugh Bernard, PhD, is an immunologist, educator, science Communicator and science advocate. Subscribe to Aimee
