A version of this story appeared in Science, Vol 380, Issue 6645.Download PDF
On a chilly holiday Monday in January 2020, a medical milestone passed largely unnoticed. In a New York City operating room, surgeons gently removed the heart from a 43-year-old man who had died and shuttled it steps away to a patient in desperate need of a new one.
More than 3500 people in the United States receive a new heart each year. But this case was different—the first of its kind in the country. “It took us 6 months to prepare,” says Nader Moazami, surgical head of heart transplantation at New York University (NYU) Langone Health, where the operation took place. The run-up included oversight from an ethics board, education sessions with nurses and anesthesiologists, and lengthy conversations with the local organization that represents organ donor families. Physicians spent hours practicing in the hospital’s cadaver lab, prepping for organ recovery from the donor. “We wanted to make sure that we controlled every aspect,” Moazami says.
That’s because this donor, unlike most, was not declared dead because of loss of brain function. He had been suffering from end-stage liver disease and was comatose and on a ventilator, with no hope of regaining consciousness—but his brain still showed activity. His family made the wrenching choice to remove life support. Following that decision, they expressed a wish to donate his organs, even agreeing to transfer him to NYU Langone Health before he died so his heart could be recovered afterward.
In individuals declared brain dead, organs can be recovered before life support is disconnected, as these people have already died; such machinery keeps organs oxygenated and healthy prior to transplant. But for this man the donation process would be altered: Life support had to be withdrawn for death to occur. His heart stopped, and his circulation with it.
As is customary regardless of whether organs will be donated, physicians waited 5 minutes to ensure that the heart didn’t start beating again on its own. It did not, and the man was declared dead. The baton then passed to the organ recovery and transplant team. They clamped blood vessels running from the torso to the brain and reconnected his body to machines that circulated oxygenated blood, causing the heart to begin pumping again.
These two interventions—initiating a heartbeat after death is declared and taking steps to prevent blood flow to the brain—are at the core of a raging debate about the ethics of such donations. To some people, the approach risks disrupting the dying process; to others, it allows that process to continue as the family desires, while also honoring individual or family wishes for organ donation.
The debate touches on the definition of death, Moazami says. “When the heart stops, we say, ‘time of death, 5:20 a.m.’” But, “The fact of the matter is, death is a process. Death is not a time point.” Cells can take hours to die. Sophisticated machinery can induce a heartbeat hours after death, but does that make a person “alive”?

Just over a year after his triple transplant, Donatelli is back to surfing and wrestling with his sons. Sandy Huffaker
An expanding number of hospitals and organ procurement organizations (OPOs), which work with donor families, support this novel category of donations, and the number performed in the U.S. is growing. “I had about 3 months, tops,” left to live, says Tony Donatelli, 41, who lives near San Diego with his wife and two young children, and who developed a rare disease that causes a dangerous buildup of protein in the body. On Valentine’s Day 2022, he became the first person in the world known to receive a heart, liver, and kidney from a donor whose organs were perfused after circulatory death. Donatelli is back to surfing, woodworking, and wrestling on the floor with his sons. “I cannot tell you how lucky I am,” he says.
Yet professional groups have expressed dueling views about the organ donation strategy, and a paper in press urges more research. Some countries are holding off on these organ donations, whereas others embrace them. One OPO says families who welcome donation do so without regard for the organ recovery technique, as such gifts can bring comfort after a terrible loss; another worries that without more research and greater attention to legal and ethical questions, there’s a risk fewer people may volunteer to be organ donors. Meanwhile, surgeons say this category of donors could increase heart transplants by up to 30%, saving lives with organs that would otherwise go unused.
“There is definitely that initial reaction that there’s something different” about this, says Anji Wall, an abdominal transplant surgeon and bioethicist at Baylor University Medical Center. Although Wall acknowledges the complexities, she supports such transplants and has performed them herself. “At the end of the day, the donor is dead,” she says. “What you do does not make them alive again.”
ORGAN TRANSPLANTATION has evolved and flourished from its first success in 1954, when a 23-year-old in Boston donated a kidney to his identical twin. In the years since, the number of transplants has surged, but demand invariably outstrips supply. In the U.S., which performs more transplants than any other country, about 104,000 people are awaiting a new organ, and, on average, 17 die each day before they get one. “We are a system that has always operated with scarcity,” says Alexandra Glazier, an attorney and president and CEO of New England Donor Services. Her system is one of 56 OPOs, each covering a geographic region across the U.S., that coordinate organ donations by working with hospitals and donor families.
The transplant system relies on public trust and the generosity of these families at an excruciating and disorienting time. In 2013, Emily Stillman, a 19-year-old college student in Michigan, was rendered brain dead by a meningitis infection. When her mother, Alicia Stillman, was approached about donating Emily’s organs, her first reaction was one of horror. “I said, ‘Absolutely not, tell them to stay away.’ … I remember screaming.”
But she quickly had second thoughts, believing her daughter would have wanted organ donation; a call to the family’s rabbi also helped. Emily’s organs were donated to five people, and the family bonded with four of them. The heart recipient, a young physician in Ohio, named his baby girl after Emily. On the anniversary of Emily’s death in February, the mothers of the heart and kidney recipients reached out to Alicia, “telling me, mother to mother, how grateful they are that they had these 10 years,” she says. Gifting Emily’s organs “was a huge part of our healing. It always gave us something positive to grab onto.”
Until recently, virtually all organ donors in the U.S. were like Emily. Following a grievous injury or some other catastrophe, they were left brain dead—which is defined as lacking any brain function, including the ability to breathe on one’s own. Their organs, however, can be protected by keeping donors on supportive machinery.
But in the 1990s, doctors grew interested in another potential category of donors: people who retained some brain activity after a serious illness or accident but who died when their circulation ceased—normally because, like the heart donor at NYU, their families had opted to withdraw life support when there was no hope of meaningful recovery. The lungs, liver, and kidneys, surgeons learned, could be recovered and function after transplant. This became known as “donation after circulatory death,” or DCD donation. Initially uncommon, the number of DCD donors has soared; today, about one-quarter of the kidneys transplanted in the U.S. are from DCD donors.
The heart was another story. Circulatory death could severely injure the organ. To address the problem, companies experimented with machinery that would run blood through a heart after it was removed from the body and stimulate its electrical activity. In 2014, Australia was the first to test one such device, made by the company TransMedics, after circulatory death. Five years later, a TransMedics clinical trial began in the U.S. Regulators there approved the system for this purpose in 2022.
Heart of the matter
Typically, organs are recovered after brain death. A newer approach allows donations from people whose relatives choose to withdraw life support, following an unsurvivable illness or injury. After death is declared due to loss of circulation, machines are connected to oxygenate the blood and allow the heart to restart, while clamps prevent blood from reaching the brain. The circulation keeps organs healthy until they are recovered for transplant.
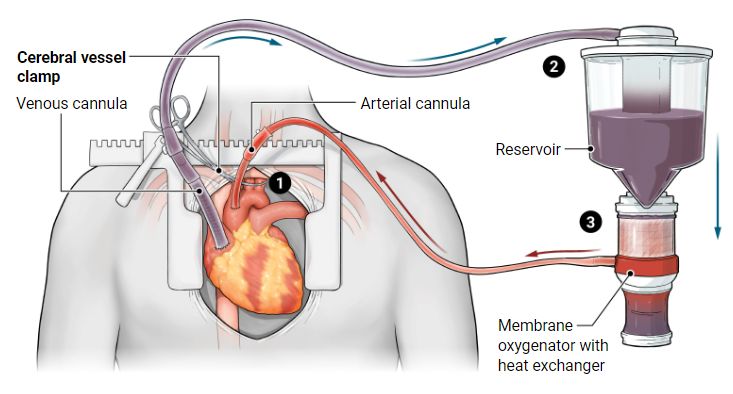
1 A clamp is placed across three arteries that supply blood to the brain, to focus perfusion on the organs being recovered and to avoid interfering with the dying process.
2 Deoxygenated blood travels from right atrium to an external reservoir.
3 Blood is warmed and oxygenated before returning to the body.
A. Mastin / SCIENCE
“It gave us access to hearts that no one else was using,” says Ashish Shah, chief of cardiac transplantation at Vanderbilt University, who participated in the trial. But using the device costs between $65,000 and $85,000 each time. Recovering organs from DCD donors can also be logistically complex, as surgeons race to remove them before they succumb to a lack of oxygen. Sometimes, one organ is recovered but another can’t be saved.
Shah, like Moazami, had been scrutinizing reports from colleagues in Europe and the United Kingdom about another kind of DCD donation. It entailed initiating oxygenated blood flow to the organs intended for transplant while they were still in the donor’s body. For the heart, that meant starting it beating again after a declaration of death.
The strategy, called normothermic regional perfusion–DCD (NRP-DCD) and sometimes abbreviated to NRP, was yielding promising results. In 2020, a team at Royal Papworth Hospital in Cambridge, England, published outcomes on three categories of heart recipients: those who received a heart from a donor after brain death, those whose heart was from a DCD donor and placed on an external device, and those whose donor organ was recovered after NRP, with perfusion within the body. All 22 people who received NRP hearts were still alive 1 year later. For the group receiving hearts maintained on external machinery, the 1-year survival rate was 86%. For people receiving hearts from donors assessed as brain dead, 1-year survival was 89%. A March study reported comparable outcomes after 157 NRP-DCD heart transplants spanning several countries and 673 heart transplants from donors declared brain dead.
These weren’t randomized trials, but nonetheless “the results were great” for NRP, says Stephen Large, a cardiothoracic surgeon at Royal Papworth. Large pioneered these heart transplants in the U.K. beginning in 2015, after years of deliberation by authorities. The inspiration, he recalls, came in 2006 when a family approached him after their 57-year-old wife and mother had suffered a devastating stroke. The relatives intended to remove life support and wished to donate her heart, but DCD heart donations weren’t possible at the time. The family reached for the next best option, asking Large whether he wanted to study her heart instead. He did, testing NRP for the first time in a human being.
IN DOING SO, Large learned that the strategy allows a surgeon to, in effect, audition the heart. You could “see how the heart was performing” in the body after restarting perfusion, he says. Moazami, the first to adopt the technique in the U.S., had the same reaction: “I can look at the pressure the heart is generating, what the chambers are doing.” In addition, surgeons believe that when oxygenated blood circulates through several organs at once, it can help them recover function lost during the dying process and their time without oxygen.
In September 2021, Moazami’s team announced that its first eight NRP-DCD heart recipients were all still alive. Earlier that year, Vanderbilt, one of the biggest heart transplant centers in the world, had launched its own NRP program. “We found ourselves traveling all over the country getting hearts,” Shah says. “There was a demand from the donor side—these families want these hearts donated. … Our job is to find a way to use” them. And use them he did. In 2022, Vanderbilt performed 40 heart transplants from NRP donors.
NRP technology is being used for other organs as well. Aleah Brubaker was a new liver transplant surgeon at the University of California, San Diego (UCSD), in the fall of 2021 when she was dispatched to get her first liver from an NRP donor. Immediately, the impact “was very evident to me,” she says. Patients are “unquestionably” getting organs more quickly, including some who might die waiting—among them Donatelli, for whom Brubaker was on the transplant team.
Heather Santiago listens to her son Jordan’s heart beat inside the person who received it. After Jordan died in a hit-and-run, his organs were donated to five people.SOUTHWEST TRANSPLANT ALLIANCE
UCSD has accepted livers from NRP donors in their late 60s, above the usual age cutoff, because perfusion inside the body helped doctors determine that the organs would be usable. Research on NRP kidney and liver recipients shows they have improved organ function and less chance of needing a second transplant than patients who get those organs via conventional DCD. “The results were much better,” says Beatriz Domínguez-Gil, director general of the Spanish National Transplant Organization. Spain has used NRP for abdominal organs for many years and began NRP heart donations in 2020. Unlike the heart, the liver and kidneys can be sustained with blood flow just to the abdomen, and without inducing a heartbeat, which eases some ethical concerns.
Transplant programs are fiercely competitive, vying for the lowest wait times and highest survival rates. At the same time, the surgeons participating in NRP donations say they wouldn’t touch them without full support from their institutions and confidence that they are ethical. The people who become NRP donors “are in this terrible state,” Shah says, most often with a devastating brain injury. They have no prospect of meaningful recovery.
“We have to put ourselves back into the context that this family has already accepted their loved one will not live and wants us to go forward with donation,” says Brad Adams, an attorney who is also president and CEO of the Southwest Transplant Alliance. His OPO oversaw seven NRP donations in the first quarter of this year compared with nine in all of 2022.
AS NRP DONATIONS ramp up in the U.S., some other countries are pumping the brakes. In the U.K., the first place to use NRP for heart donation, they ground to a halt in 2019. Concern bubbled up at a meeting between U.K. and Canadian physicians about whether, despite the clamping of vessels to the brain before organ recovery, some blood could still reach it.
In an attempt to study this, Large and his colleagues examined three NRP donors, looking for blood in tiny arteries that thread up to large vessels feeding the brain. In one person, there was detectable blood flow in these vessels, estimated at 50 milliliters per minute, about 7% of the normal rate. Whether blood actually reached the brain wasn’t tested. “There was a heated argument” among physicians, Large says, about how much blood, if any, could get to the brain, and its significance.
Following Large’s observations, the donation and transplant communities paused NRP for heart donations in the U.K. The country continues to support NRP for liver and kidney donors, as vessels there are clamped lower in the abdomen and the chance of blood reaching beyond the torso is considered remote.
A knotty and mind-bending question is whether such flow would matter. “The clamping of the vessels is … a postmortem intervention,” says Marat Slessarev, a specialist in critical care and organ donation at Western University in Canada. Like colleagues who work in intensive care units, he’s comfortable with the standard of declaring death 5 minutes after the heart stops following a withdrawal of life support. A recent study that included 480 patients whose life support was withdrawn backs this up. Transient heart activity resumed spontaneously in 67, but the longest lag time was 4 minutes and 20 seconds, investigators reported in The New England Journal of Medicine in 2021.
But could blood flow to the brain after circulatory death still spark brain activity or function? Because dying is a process, “the best way to put it is, we don’t know,” Slessarev says. He wants to prove that circulatory death guarantees rapid brain death, which he suspects is the case, and that clamping guarantees zero blood flow to the brain.
Slessarev and colleagues began to address the first challenge in a pilot study of eight people after the withdrawal of life support. They found that brain activity actually ceased before the heart stopped beating—on average, 78 seconds earlier. “Blood pressure falls below a certain level, then the brain stops, then circulation stops,” Slessarev says. “That’s sort of the sequence of events.” To see whether these results hold up, he’s now co-leading a larger effort across Canada that aims to enroll about 90 people. His goal is to inform organ donation policies in his country, which does not permit NRP donations but is weighing them.
Slessarev is also co-leading a Canadian team preparing to probe whether there’s any blood flow to the brain after clamping. He’s reassured by a January 2022 paper from a team in Denmark, showing that in pigs, 8 minutes without circulation followed by clamping prevented all blood flow and brain activity when the heart restarted. Animals that did not receive any clamping showed brain waves on monitors.
Others are conducting similar studies. Moazami recently hunted for cerebral blood flow with a transcranial doppler machine in two NRP heart donors. “We could not detect any,” he says, and he plans to examine this in more cases.
In the U.K., a team at Royal Papworth will study NRP donors in the coming months, using a test called a CT angiogram to see whether any blood appears in cerebral arteries and returns through the veins. The latter could indicate perfusion through tissues. “I will be really surprised” if there is cerebral blood flow to any meaningful degree, says Antonio Rubino, an intensivist at the hospital who is leading the trial. But he still wants the work to be done.
FOR NOW, “the global ethics of this have not been resolved,” Moazami says, and even in the U.S., controversy is erupting. “The definition of death is kind of broken,” says Brendan Parent, an NYU bioethicist who helped his hospital and several others consider the ethics of NRP. Irreversible loss of either all circulation or all brain function qualifies as death in the U.S. But circulation can, in theory, be reinstituted by machinery even many hours after a death, Parent notes, rendering the circulatory death definition, practically speaking, meaningless.
As NRP donations increased in the U.S., debate emerged in spurts and crescendoed this spring. An early salvo came in April 2021, when the American College of Physicians, which represents internal medicine doctors, deemed NRP unethical in part because it can reestablish a heartbeat. Wall, Shah, and others published a response arguing that NRP meets ethical standards for consent and organ donation. On the legal front came a volley from Alexander Capron, a law professor at the University of Southern California, and Glazier of the New England OPO, arguing that NRP is inconsistent with U.S. legal standards because it involves restarting circulation whose permanent absence prompted the declaration of death in the first place. Adams, Parent, and others shot back that NRP is consistent with U.S. legal standards of death, because the technique is limited to perfusing organs and doesn’t impact the determination of death. Glazier, who says her OPO is one of a few that don’t yet permit NRP for the organ recoveries they coordinate, emphasizes that her concerns are more about a “misalignment rather than a big violation.” Can these potential donors return to what is considered meaningful life with NRP interventions? “Absolutely not,” she says. But she still has significant concerns about the strategy.
Glazier thinks death in the U.S. should be redefined as a permanent loss of brain function, period, with no separate definition for circulatory death. She joined authors from eight countries, including some, such as France and Spain, where NRP has long been practiced, on a paper in press in Transplantation. They urged all countries to adopt a brain-based definition of death, which could be determined by a permanent absence of circulation to the brain. This would ease concerns about NRP if studies like those planned in Canada and the U.K. confirm that clamping prevents any blood flow to the brain.
In March, the U.S. National Institutes of Health and the Organ Donation and Transplantation Alliance held separate meetings to discuss NRP. Interest in the topic was so great that the alliance was forced to find a larger venue. Elizabeth Pomfret, chief of transplant surgery at the University of Colorado School of Medicine, which recovers organs with NRP, welcomes the discussion. But Pomfret, who is president-elect of the American Society of Transplant Surgeons, worries about “confusion that’s arisen around these questions of the permanence of death. … This whole conversation is sort of reeling out of control.”
Parent stresses that protecting the potential donor’s interests is the highest priority. But he adds, “It’s not as simple as just trying harder to save that person’s life,” because doing so can become futile and cause more suffering. For individuals who expressed a wish to be a donor and whose life cannot be saved, “what would be in their best interest is preserving their organs” for others who need them, he says. Parent is now developing a project to study public and donor family perceptions of NRP.
Alicia Stillman and her husband didn’t have to make a decision about NRP donation for Emily. But if they had, she imagines that the distinction between circulatory and brain death would not have been so important to her. “Nothing was going to bring Emily back,” she says. “There was nothing I could have done to save her.” Donor families “should be at the head of the table” helping make these decisions, she believes.
Across the country in Texas, another family agrees. Andrew Santiago doesn’t know what he and his wife Heather Santiago would have done in this situation, but he suggests these donations “be positioned as an optional deal,” with the family making that call. His 25-year-old son Jordan was left brain dead almost 3 years ago after a hit-and-run. Jordan’s organs, like Emily’s, were donated to five people, and “that’s what’s keeping us going,” his mother says. Both the Stillmans and the Santiagos launched foundations that include organ donor advocacy.
Early next month, the American Transplant Congress will convene in San Diego, about a 15-minute drive from Donatelli’s home near the Pacific Ocean; he may be speaking there. Pomfret, meanwhile, is organizing a symposium for the conference’s opening day to develop a formal position statement on NRP and lay plans for national data collection on NRP cases and universal standards for recovering those organs, such as clamping techniques. “We’re showing the community that we’re responsible,” Pomfret says. “This isn’t just a free-for-all.”
UCSD Jacobs Medical Center, the site of the institute’s transplant surgeries, is a short drive up the coast from the convention center where surgeons will gather. In one of the hospital’s beds, another triple-organ transplant patient is currently recovering. His new heart, liver, and kidney were given by an NRP donor.
Jennifer Couzin-Frankel is a reporter at Science, covering biomedical research.
Science has been at the center of important scientific discovery since its founding in 1880—with seed money from Thomas Edison. Today, Science continues to publish the very best in research across the sciences, with articles that consistently rank among the most cited in the world. In the last half century alone, Science published:
- The entire human genome for the first time
- Never-before seen images of the Martian surface
- The first studies tying AIDS to human immunodeficiency virus
Science is more than just a magazine. Subscribers, like you, make up a community that’s dedicated to driving scientific progress that benefits everyone. When you join AAAS, you’re directly helping us promote diversity in STEM, support educational opportunities for underserved communities, engage policymakers on issues like climate change, and much more. Become a Member.


Spread the word