On a December afternoon, 13 days before the winter solstice, six men and women checked into the Surrey Clinical Research Facility, part of the University of Surrey in the United Kingdom. After having their noses swabbed to check for 16 different respiratory viruses, they walked into their own temperature-regulated rooms and, for 24 hours, each person stayed in a semirecumbent position in dim light. Nurses placed a cannula into a vein of each person’s arm, allowing easy sampling of blood that flowed through a tube to portals in the wall. The six subjects could press buzzers for bathroom breaks, where the stool and urine were collected, but otherwise, they were alone in the near-dark.
None of these people were sick. And although the shortest day of the year was approaching, their ritual had nothing to do with pagan rites, Yuletide traditions, or the annual hippie gathering at nearby Stonehenge to celebrate the rebirth of the Sun. Instead, they were paid volunteers in a study led by infectious disease ecologist Micaela Martinez of Columbia University to investigate a phenomenon recognized 2500 years ago by Hippocrates and Thucydides: Many infectious diseases are more common during specific seasons. “It’s a very old question, but it’s not very well studied,” Martinez says.
It’s also a question that has suddenly become more pressing because of the emergence of COVID-19. With SARS-CoV-2, the virus that causes the disease, now infecting more than 135,000 around the globe, some hope it might mimic influenza and abate as summer arrives in temperate regions of the Northern Hemisphere, where about half of the world’s population lives. U.S. President Donald Trump has expressed that hope repeatedly. “There’s a theory that, in April, when it gets warm—historically, that has been able to kill the virus,” Trump said on 14 February. But what’s known about other diseases doesn’t offer much support for the idea that COVID-19 will suddenly disappear over the next few weeks.
Different diseases have different patterns. Some peak in early or late winter, others in spring, summer, or fall. Some diseases have different seasonal peaks depending on latitude. And many have no seasonal cycle at all. So no one knows whether SARS-CoV-2 will change its behavior come spring. “I would caution over-interpreting that hypothesis,” Nancy Messonnier, the point person for COVID-19 at the U.S. Centers for Disease Control and Prevention, said at a press conference on 12 February. If the seasons do affect SARS-CoV-2, it also could defy that pattern in this first year and keep spreading, because humanity has not had a chance to build immunity to it.
Even for well-known seasonal diseases, it’s not clear why they wax and wane during the calendar year. “It’s an absolute swine of a field,” says Andrew Loudon, a chronobiologist at the University of Manchester. Investigating a hypothesis over several seasons can take 2 or 3 years. “Postdocs can only get one experiment done and it can be a career killer,” Loudon says. The field is also plagued by confounding variables. “All kinds of things are seasonal, like Christmas shopping,” says epidemiologist Scott Dowell, who heads vaccine development and surveillance at the Bill and Melinda Gates Foundation and in 2001 wrote a widely cited perspective that inspired Martinez’s current study. And it’s easy to be misled by spurious correlations, Dowell says.
Despite the obstacles, researchers are testing a multitude of theories. Many focus on the relationships between the pathogen, the environment, and human behavior. Influenza, for example, might do better in winter because of factors such as humidity, temperature, people being closer together, or changes in diets and vitamin D levels. Martinez is studying another theory, which Dowell’s paper posited but didn’t test: The human immune system may change with the seasons, becoming more resistant or more susceptible to different infections based on how much light our bodies experience.
Beyond the urgent question of what to expect with COVID-19, knowing what limits or promotes infectious diseases during particular times of year could point to new ways to prevent or treat them. Understanding seasonality could also inform disease surveillance, predictions, and the timing of vaccination campaigns. “If we knew what suppressed influenza to summertime levels, that would be a lot more effective than any of the flu vaccines we have,” Dowell says.
The calendar of epidemics
At least 68 infectious diseases are seasonal, according to a 2018 paper by Micaela Martinez of Columbia University. But they’re not in sync, and seasonality varies by location. In this graphic, based on U.S. federal and state health records, each bubble represents the percentage of annual cases that occurred in each month. (The data are old because many diseases declined—in some cases to zero—after introduction of vaccines.)
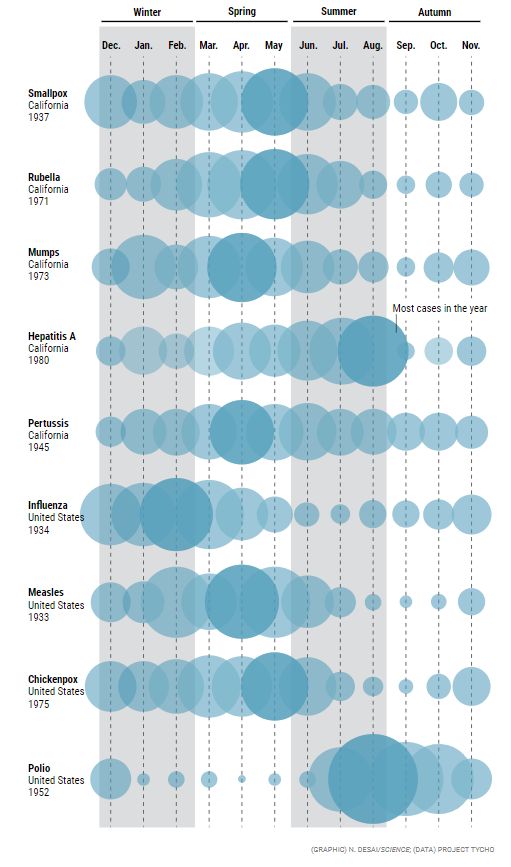
MARTINEZ BECAME interested in seasonality when, as an undergraduate at the University of Alaska Southeast, she had a job tagging Arctic ringed seals, doing skin biopsies and tracking their daily and seasonal movements. While working on her Ph.D., her focus on seasonality shifted to polio, a much-feared summer disease before the advent of vaccines. (Outbreaks often led to the closing of swimming pools, which had virtually nothing to do with viral spread.) Polio seasonality in turn made her curious about other diseases. In 2018, she published “The calendar of epidemics” in PLOS Pathogens, which included a catalog of 68 diseases and their peculiar cycles.
Except in the equatorial regions, respiratory syncytial virus (RSV) is a winter disease, Martinez wrote, but chickenpox favors the spring. Rotavirus peaks in December or January in the U.S. Southwest, but in April and May in the Northeast. Genital herpes surges all over the country in the spring and summer, whereas tetanus favors midsummer; gonorrhea takes off in the summer and fall, and pertussis has a higher incidence from June through October. Syphilis does well in winter in China, but typhoid fever spikes there in July. Hepatitis C peaks in winter in India but in spring or summer in Egypt, China, and Mexico. Dry seasons are linked to Guinea worm disease and Lassa fever in Nigeria and hepatitis A in Brazil.
Seasonality is easiest to understand for diseases spread by insects that thrive during rainy seasons, such as African sleeping sickness, chikungunya, dengue, and river blindness. For most other infections, there’s little rhyme or reason to the timing. “What’s really amazing to me is that you can find a virus that peaks in almost every month of the year in the same environment in the same location,” says Neal Nathanson, an emeritus virologist at the University of Pennsylvania Perelman School of Medicine. “That’s really crazy if you think about it.” To Nathanson, this variation suggests human activity—such as children returning to school or people huddling indoors in cold weather—doesn’t drive seasonality. “Most viruses get transmitted between kids, and under those circumstances, you’d expect most of the viruses to be in sync,” he says.
Nathanson suspects that, at least for viruses, their viability outside the human body is more important. The genetic material of some viruses is packaged not only in a capsid protein, but also in a membrane called an envelope, which is typically made of lipids. It interacts with host cells during the infection process and helps dodge immune attacks. Viruses with envelopes are more fragile and vulnerable to adverse conditions, Nathanson says, including, for example, summertime heat and dryness.
A 2018 study in Scientific Reports supports the idea. Virologist Sandeep Ramalingam at the University of Edinburgh and his colleagues analyzed the presence and seasonality of nine viruses—some enveloped, some not—in more than 36,000 respiratory samples taken over 6.5 years from people who sought medical care in their region. “Enveloped viruses have a very, very definite seasonality,” Ramalingam says.

In a study in New York and New Jersey, Micaela Martinez hopes to find out how artificial lighting affects the immune system. MIKE GRIPPI
RSV and human metapneumovirus both have an envelope, like the flu, and peak during the winter months. None of the three are present for more than one-third of the year. Rhinoviruses, the best-known cause of the common cold, lack an envelope and—ironically—have no particular affinity for cold weather: The study found them in respiratory samples on 84.7% of the days of the year and showed that they peak when children return to school from summer and spring holidays. Adenoviruses, another set of cold viruses, also lack an envelope and had a similar pattern, circulating over half the year.
Ramalingam’s team also studied the relationship between viral abundance and daily weather changes. Influenza and RSV both did best when the change in relative humidity over a 24-hour period was lower than the average (a 25% difference). “There’s something about the lipid envelope that’s more fragile” when the humidity changes sharply, Ramalingam concludes.
Jeffrey Shaman, a climate geophysicist at Columbia, contends that what matters most is absolute humidity—the total amount of water vapor in a given volume of air—and not relative humidity, which measures how close the air is to saturation. In a 2010 paper in PLOS Biology, Shaman and epidemiologist Marc Lipsitch of the Harvard T.H. Chan School of Public Health reported that drops in absolute humidity better explained the onset of influenza epidemics in the continental United States than relative humidity or temperature. And absolute humidity drops sharply in winter, because cold air holds less water vapor.
Why lower absolute humidity might favor some viruses remains unclear, however. Variables that could affect the viability of the viral membrane could include changes in osmotic pressure, evaporation rates, and pH, Shaman says. “Once you get down to the brass tacks of it, we don’t have an answer.”
Will SARS-CoV-2, which has an envelope, prove fragile in spring and summer, when absolute and relative humidity climb? The most notorious of the other coronavirus diseases, SARS and Middle East respiratory syndrome (MERS), offer no clues. SARS emerged in late 2002 and was driven out of the human population in the summer of 2003 through intensive containment efforts. MERS sporadically jumps from camels to humans and has caused outbreaks in hospitals, but never widespread human-to-human transmission like COVID-19. Neither virus circulated for long enough, on a wide enough scale, for any seasonal cycle to emerge.
Four human coronaviruses that cause colds and other respiratory diseases are more revealing. Three have “marked winter seasonality,” with few or no detections in the summer, molecular biologist Kate Templeton, also at the University of Edinburgh, concluded in a 2010 analysis of 11,661 respiratory samples collected between 2006 and 2009. These three viruses essentially behave like the flu.
That does not mean COVID-19 will as well. The virus can clearly transmit in warm, humid climates: Singapore has more than 175 cases. Two new papers published on preprint servers this week come to opposite conclusions. One, co-authored by Lipsitch, looked at COVID-19 spread in 19 provinces across China, which ranged from cold and dry to tropical, and found sustained transmission everywhere. The second study concludes that sustained transmission appears to occur only in specific bands of the globe that have temperatures between 5°C and 11°C and 47% to 70% relative humidity.
In the final analysis, there’s a balancing act between environmental factors and a population’s immune system. The other coronaviruses have long been around, so a certain part of the population has immunity, which may help exile those viruses under unfavorable conditions. But that’s not true for COVID-19. “Even though there might be a big seasonal decline, if enough susceptible people are around, it can counter that and continue for a long time,” Martinez says. Lipsitch doesn’t think the virus will go poof in April either. Any slowdown “is expected to be modest, and not enough to stop transmission on its own,” he wrote in a recent blog post.
IN SURREY, MARTINEZ is investigating a different factor that might eventually affect COVID-19 incidence. Her subjects have returned to the clinic repeatedly—at the winter and summer solstices and again at the spring and autumn equinoxes—so the researchers can evaluate how their immune system and other physiology change over the course of the day and from season to season.
She doesn’t expect to show that our immunity is, say, weaker in the winter and stronger in the summer. But by counting different immune system cells, assessing metabolites and cytokines in the blood, deciphering the fecal microbiome, and measuring hormones, Martinez’s team hopes to find that the seasons may “restructure” the immune system, making some types of cells more abundant in certain locales, and others less, in ways that influence our susceptibility to pathogens.
Animal studies support the idea that immunity changes with the seasons. Ornithologist Barbara Hall from the University of Groningen and her colleagues, for example, studied European stonechats, small songbirds that they caught and then bred in captivity. By taking multiple blood samples over the course of 1 year, they found that the birds ramp up their immune systems in the summer, but then tamp them down in the autumn, the time they migrate, presumably because migration is a big drain on their energy.
Melatonin, a hormone primarily secreted at night by the pineal gland, is a major driver of such changes. Melatonin keeps track of the time of day but is also a “biological calendar” for the seasons, says Randy Nelson, an endocrinologist at the University of West Virginia who specializes in circadian rhythms. When nights are long, more melatonin is released. “The cells say, ‘Oh, I’m seeing quite a bit of melatonin, I know, it’s a winter night.’” In studies of Siberian hamsters—which, unlike mice, are diurnal—Nelson and his co-workers have shown that administering melatonin or altering light patterns can change immune responses by up to 40%.

Seasonal changes in humidity, temperature, and other factors may affect the viability of viruses in droplets produced when people sneeze or cough. NICK GREGORY/ALAMY STOCK PHOTO
The human immune system, too, seems to have an innate circadian rhythm. For instance, a vaccine trial in 276 adults by researchers at the University of Birmingham randomly assigned half to receive an influenza vaccine in the morning and the other half in the afternoon. Participants in the morning group had significantly higher antibody responses to two of the three flu strains in the vaccine, the researchers reported in 2016.
There’s evidence of seasonal variation in the actions of human immune genes as well. In a massive analysis of blood and tissue samples from more than 10,000 people in Europe, the United States, Gambia, and Australia, researchers at the University of Cambridge found some 4000 genes related to immune function that had “seasonal expression profiles.” In one German cohort, expression in white blood cells of nearly one in four genes in the entire genome differed by the seasons. Genes in the Northern Hemisphere tended to switch on when they were switched off south of the equator, and vice versa.
Just how these massive changes affect the body’s ability to fight pathogens is unclear, however, as immunologist Xaquin Castro Dopico and colleagues explain in a 2015 paper describing the findings. And some changes could be the result of an infection, instead of the cause. The team tried to eliminate people who had acute infections, but “of course a seasonal infectious burden likely plays a part,” says Dopico, who now is at the Karolinska Institute. And seasonal immunity changes could not explain all the complex variation in seasonality that diseases show. “They’re all out of sync with each other,” Nathanson points out. He’s also skeptical that seasonal immune system changes could be large enough to make a difference. “It would have to be pretty markedly different.”
Martinez, however, says she has found intriguing hints. Early analyses from her Surrey study, which ended collecting data in December 2019, don’t reveal anything about seasonality yet, but they do show that specific subsets of white blood cells that play central roles in immune system memory and response are elevated at certain times of day. She hopes to firm up the finding by launching a similar but larger study next year.
Martinez cautions, however, that artificial light may play havoc with the circadian rhythms that have evolved, with unpredictable effects on disease susceptibility. To explore possible impacts, Martinez has a separate study underway, with Helm, in both urban and rural parts of New York and New Jersey. They have installed light sensors on trees and poles and outfitted participants with devices that monitor light exposure and body temperature. “The fact that people really are just kind of washing out the rhythms in light exposure can be problematic,” she says.
“EXPERIMENTS OF NATURE” could also offer insights into the factors affecting disease seasonality, Dowell suggested in his 2001 paper. People from the Southern and Northern Hemispheres who have adapted to different seasons regularly mix on cruise ships or at conventions, where they are confronted by the same pathogens. Witness the massive COVID-19 outbreak on the Princess Diamond, which was docked and quarantined in Yokohama, Japan, for 2 weeks last month: Researchers could potentially analyze whether they were infected at different rates.
Whatever the answers, they might eventually bring important public health benefits, Martinez says. For example, “If we know how best to administer vaccines, in terms of what time of year and the best time of day to take advantage of our immune systems, then we can get a lot more bang for our buck,” she says.
The global COVID-19 emergency may bring more attention to the research and help speed discoveries, she says. But for now, no one knows whether rising humidity, the lengthening days, or some as-yet-unsuspected seasonal effect will come to the rescue—or whether humanity must confront the pandemic without any help from the seasons.
Time will tell.
Jon Cohen is a staff writer for Science.


Spread the word