Every day, millions of people wake up to fight battles they never chose— against cancer cells multiplying in their bodies, immune systems attacking their own tissues, or allergens that turn simple meals into life-threatening encounters. What if we told you that your body already holds the key to fighting and possibly curing disease, waiting only for science to unlock it?
Scientific discovery and innovation are about reimagining what’s possible for human health. For centuries, medicine has fought illness with external tools such as scalpels, pills, and radiation. But a new frontier is emerging—one that doesn’t just treat disease, but empowers the body to fight back through harnessing its own internal defense system— the immune system. Let’s discuss…
The Immune System is a Mobile Defense System
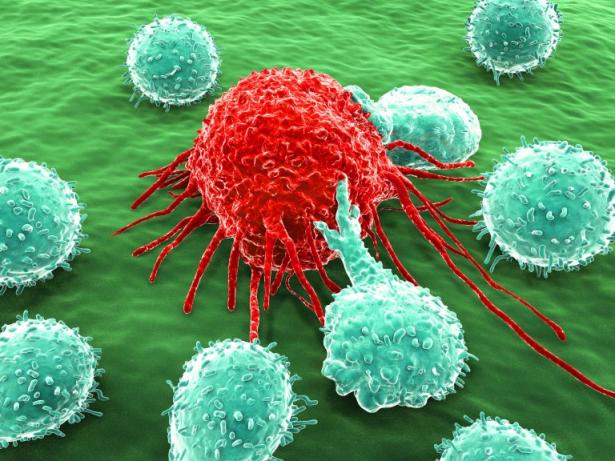
The immune system is the body’s built-in mobile defense system— a complex network of cells, tissues, and organs that work together to protect the body from harmful invaders like bacteria, viruses, and even rogue cells like cancer. The cells of the immune system are continually on patrol, traveling throughout your body looking for infectious invaders and damage. Think of the immune system as a mobile defense system, like dialing 911 to call in the first responders. When a threat is detected, immune cells rush to the scene, assess the danger, and take action to neutralize and eliminate it.
But just like any emergency response team, the immune system needs to act with the right intensity at the right time. That’s why balance is key. When it’s underactive, we’re more vulnerable to infections and slow to heal. But when it’s overactive, it can mistakenly attack our own tissues, leading to autoimmune diseases and chronic inflammation.
Immunotherapy: Resetting the Thermostat of the Immune System
Immunotherapy is transforming how we think about fighting disease. It is an innovative approach in medicine that works by harnessing the body’s own immune system to fight disease. You can think of it like a thermostat for your immune system—it can be turned up to boost the body’s defenses, dialed down to reduce inflammation, or redirected to focus the immune system on specific targets or threats. In certain cancers, for example, it can redirect immune cells to hunt down tumors. In autoimmune conditions like rheumatoid arthritis, it can suppress the immune system’s misguided attacks on healthy tissue. And in allergies, it can help retrain the immune system to tolerate rather than attack. By fine-tuning the immune response, immunotherapy offers a powerful, personalized way to treat a wide range of diseases, often with fewer side effects than traditional therapies.
Immunotherapy is Changing the Way We Fight Disease
While immunotherapy is most well-known for treating cancer, where treatments like checkpoint inhibitors or CAR T cell therapy have given patients with advanced or previously untreatable cancer a fighting chance or even complete remission, its potential extends far beyond oncology to autoimmune diseases, allergies, and infectious diseases such as COVID-19.
Historically, most of these diseases were treated with medications that broadly suppressed the immune system or destroyed rapidly dividing cells. For example, chemotherapy kills not only the cancerous cells, but also the healthy ones, leading to side effects like nausea, fatigue, or even hair loss. Steroids and immunosuppressants, typically used to treat autoimmune disorders, can help subdue inflammation, but this comes at the expense of making patients more susceptible to infections and prone to other complications.
On the other hand, immunotherapy takes a more targeted and individualized approach. It trains and reprograms the immune system to directly target the problem, whether it’s a cancer cell, an autoimmune signal, or an immune trigger gone awry, while sparing the entire system. This translates to fewer side effects, more effective disease control, and, in some cases, long-term protection after the treatment ends.
The clinical impact is already life-altering. Cancer patients who had months to live are now surviving for years. Children with life-threatening food allergies are able to tolerate certain foods again after undergoing immunotherapy. Patients with autoimmune disorders are now finding relief without relying on daily high-dose medications. Immunotherapy is a game changer that equips the immune system to do what it was always designed to do: defend and mend.
Monoclonal Antibodies are a Precise Tool in the Immunotherapy Arsenal
Monoclonal antibodies are some of the most powerful weapons in immunotherapy. These are laboratory-made antibody proteins that can mimic the immune system's response, with the power to attack the body’s invaders, similar to immune B cells that produce them, called plasma cells. Natural antibodies have a broader range of activity, but monoclonal antibodies are engineered to bind exclusively to one chosen target, giving them exceptional specificity. Think of it as target-seeking missiles, which guide the immune system in blowing up what needs to be destroyed. The impact of these antibodies on modern medicine is profound and can't be stressed enough. Trastuzumab (Herceptin) transformed breast cancer treatment by selectively targeting HER2-positive tumors. Adalimumab (Humira) revolutionized the treatment of autoimmune diseases, like arthritis, by neutralizing the action of TNF-alpha. Ipilimumab (Yervoy) became one of the first successful checkpoint inhibitors in advanced melanoma by blocking CTLA-4. This type of therapy doesn't just address the symptoms; it navigates the immune system to a brand new roadmap of what we can do for these chronic and deadly diseases.
Turning the ‘Heat’ of the Immune System Up – Fighting Cancer
Immune Checkpoint Inhibitors
Checkpoint blockade, also called immune checkpoint inhibition, is a powerful form of cancer immunotherapy that uses monoclonal antibodies to "release the brakes" on the immune system. These antibodies target proteins like PD-1 and CTLA-4, which act as "checkpoints" that normally keep immune responses by T cells in check to prevent damage to healthy tissue. Checkpoint molecules (PD-1 and CTLA-4) exist to prevent autoimmune attacks on self tissue. As an example, when the ligand for PD-1 (PD-L1) on the surface of T cells binds to PD-1 on a cell in the body, such as a skin cell, this interaction prohibits the T cell from engaging in an attack against the self cell. However, cancer cells, which are derived from self tissue - often utilize these checkpoints to avoid being attacked.
Immunotherapeutic drugs such as nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) block these checkpoint proteins, allowing T cells to stay active and attack tumors more effectively. This approach has led to major advances in treating cancers like melanoma, lung cancer, and kidney cancer. While checkpoint blockade can be life-saving, it can also cause side effects when the same T cells that have been ‘released’ become overactive, sometimes attacking healthy organs. These side effects can range from mild (like fatigue or skin rash) to more serious issues such as inflammation of the lungs, liver, or intestines. Still, for many patients, this therapy offers a powerful new way to fight cancer using their own immune system.
Chimeric Antigen Receptor T cells (CAR T cells)
CAR T cells are a cutting-edge form of immunotherapy that reprograms a patient’s own T cells to recognize and destroy a specific target, such as a protein on the surface of cancer cells. To create this personalized medicine, scientists collect T cells from a patient’s blood and modify them in the lab to produce chimeric antigen receptors—called CARs—that recognize and latch onto specific proteins on the surface of cancer cells. Once these engineered T cells are infused back into the patient, they act like guided missiles, seeking out and killing cancer cells with precision. CAR T cell therapy has shown remarkable success in treating certain blood cancers, such as acute lymphoblastic leukemia (ALL). While this therapy can be highly effective, it is important to closely monitor patients during and after treatment for serious side effects, including cytokine release syndrome and neurologic symptoms.
Turning the ‘Heat’ of the Immune System Down – Treating Autoimmune Diseases
Autoimmune diseases occur when the immune system, which normally protects us from harmful invaders, mistakenly identifies the body’s own cells as threats and launches an attack. This internal misfire can lead to chronic inflammation and damage in tissues like joints, nerves, or organs—hallmarks of conditions such as rheumatoid arthritis, multiple sclerosis, and type 1 diabetes. Immunotherapy offers a promising way to “dial down” this overreaction by retraining or redirecting the immune response. For example, monoclonal antibodies can block inflammatory signals that drive autoimmune damage, helping to ease symptoms and slow disease progression. In rheumatoid arthritis, these therapies target and neutralize pro-inflammatory molecules like TNF-alpha, while in multiple sclerosis, they can be used to reduce immune attack on the nervous system.
More recently, researchers have begun exploring CAR T-cell therapy—originally developed for cancer—for autoimmune diseases like systemic lupus erythematosus. In this setting, CAR T-cells may help reset the immune system by targeting the autoantibody-producing B cells causing systemic disease. While B cells typically produce protective antibodies that target pathogenic invaders, autoreactive B cells, that mistakenly identify self components as foreign, fuel the immune attack by producing autoreactive antibodies (aka autoantibodies) that target cells within the body for destruction. In fact, CAR T cells that target B cells, specifically a B cell surface marker called CD19, have shown remarkable results in early trials for diseases like systemic lupus erythematosus and even rare conditions like Stiff Person Syndrome. The pathogenesis of these autoimmune diseases is driven by the production of autoantibodies. These breakthroughs are reshaping how we think about treating autoimmune conditions—not just managing symptoms, but potentially resetting the immune system thermostat.
Immunotherapy is the Future of Medical Innovation and Impact
The future of immunotherapy is already here. What began as a cancer therapy breakthrough is now expanding to other applications outside of oncology. Researchers are applying immunotherapy to the treatment of chronic infections like HIV and hepatitis, autoimmune diseases such as lupus, type 1 diabetes, psoriasis, and inflammatory bowel diseases, and even neurodegenerative conditions like Alzheimer’s, Parkinson’s, and multiple sclerosis. For the treatment of allergies, researchers are also working on therapies that would retrain the immune system to tolerate harmless triggers, such as food proteins or pollen, rather than attacking them. These recent developments are a result of a deeper understanding of how immune dysfunction is a common denominator of numerous health issues once regarded as unrelated.
Current research could open up new possibilities in the world of immunotherapy. Personalized cancer vaccines, for example, are already in clinical trials. They prime a person’s immune system to make it recognize and destroy their own individual tumor markers. Combination strategies, such as pairing immunotherapy with chemotherapy, radiation, and/or targeted therapy, can improve efficacy and reduce resistance. Novel biomarker discovery and single-cell immune profiling will continue to allow for personalized treatment approaches based on one’s own unique immune signature. The product is a safer and more accurate medication. This is indicative of the evolving nature and progress of healthcare, from conventional to immune-based precision medicine. So, what is the ultimate end goal? To do more than symptom management and treat and eventually cure diseases previously considered beyond reach.
Immunotherapy represents more than innovation; it represents possibility
Immunotherapy is revolutionizing the way we understand and treat disease, not by simply boosting the immune system, but by learning how to guide it with precision. Sometimes that means turning the immune response up to fight cancer or infections; other times, it means dialing it down or even switching it off to calm autoimmune attacks. From monoclonal antibodies that ease chronic inflammation to CAR T-cell therapies that reprogram the cells of the immune system itself, the real-world impact is profound and deeply personal.
This nuanced approach is transforming medicine, offering new hope to patients with conditions once thought untreatable. But unlocking that potential will rely on further scientific research and funding. Every breakthrough we've discussed, from cancer to autoimmune disease, is the product of decades of investment in basic science. To keep pushing boundaries, we have to continue supporting the science that generates tomorrow's cures.
For patients and families facing these challenges, immunotherapy represents more than innovation— it represents possibility. It's the difference between managing a disease and potentially conquering it, between borrowed time and reclaimed futures. As we continue to unlock the language of our own immune systems, we're not just advancing medicine; we're expanding what it means to hope. We think that’s pretty incredible.
Stay Curious,
Unbiased Science
Jess Steier, DrPH Public health scientist, host of Unbiased Science, and quirky and empathetic science communicator.
Aimee Pugh Bernard, PhD Immunologist | Educator | Science Communicator | Science Advocate
Christy Kestner, PhD, MS Neuroimmunologist. Science Writer. Science Communicator. Founder of Brain & Beyond. Reader. Athlete. Hodophile. NOT medical advice.
Want to support our work? The best way is to subscribe to our Substack and share our content. While all our articles are always completely free to read, paid subscriptions help sustain our in-depth reporting on public health and science topics. Thank you for considering it!

New Clue to How Matter Outlasted Antimatter at the Big Bang Is Found
Kenneth Chang
New York Times
Physicists working at the CERN particle physics lab said they detected a slight but significant difference in how particles of matter and antimatter decay.
July 16, 2025

Spread the word