Sunday Science: Giant Project Will Chart Human Immune Diversity To Improve Drugs and Vaccines
The hepatitis B vaccine is one of the most potent immunizations, usually providing decades of protection against the deadly liver virus. But in about 10% of people it doesn’t work, and in 2020, Amy Huei-Yi Lee, a systems biologist at Simon Fraser University, and her colleagues set out to determine whether they could predict who would benefit. The scientists found that data on recipients’ immune systems such as the abundance of certain proteins and the activity patterns of a few genes foretold whether they would generate defenses against the virus. “We got a sense of what factors drive the vaccine response and what ,” Lee says.
She and her colleagues were only able to take measurements from a handful of patients, but an ambitious effort slated to begin early this year will collect such data from hundreds of thousands of volunteers throughout the world. Called the Human Immunome Project (HIP) and backed by an international consortium of companies, government agencies, and universities, the effort will probe thousands of immune variables in blood and tissue samples. The result will likely be the world’s largest and most comprehensive immunological database, a resource for scientists investigating immune system differences and how they influence our responses to vaccines and drugs and our vulnerability to illness. “There’s a huge opportunity here in terms of understanding human disease,” says immunologist Mark Davis of Stanford University, who is not involved in the project.
And that’s just the start for the effort, which currently operates on about $5 million a year in funding but could ultimately cost billions. An offshoot of a previous effort known as the Human Vaccines Project, HIP will also use the data as fodder for new artificial intelligence (AI) models that could predict immune system responses across entire populations, providing valuable insights not just for pharmaceutical companies and governments, but even for doctors and patients. “The impacts will be felt globally,” says neuroscientist Hans Keirstead, the Irvine, California–based project’s CEO.
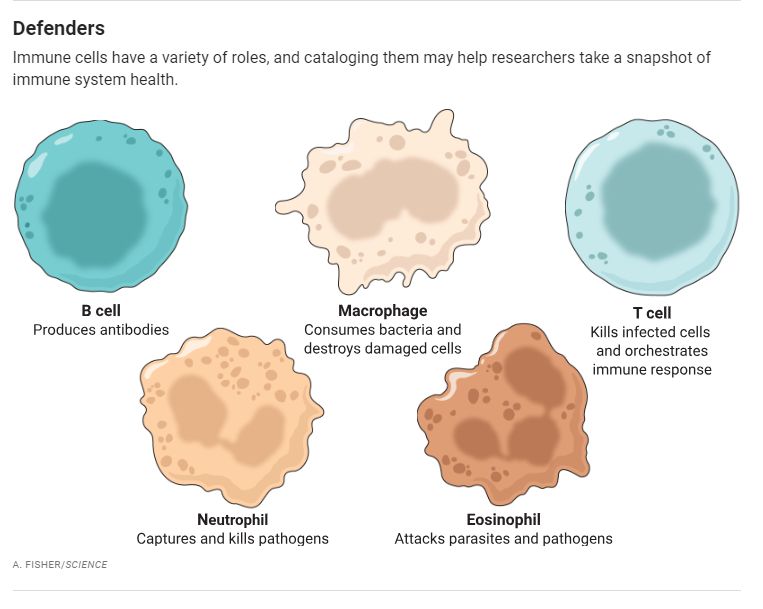
Scientists unconnected to the project say its goal of compiling a basic immune database for the world is feasible. “We have the experience and technology,” says immunologist Allison Greenplate of the University of Pennsylvania. But she and others question how much insight AI will add. “There is a lot of low-hanging fruit we don’t need AI to pick” but that researchers can parse themselves, says immunologist Paul Thomas of St. Jude Children’s Research Hospital.
In the field of cardiology, a lipid panel reveals a lot about a patient’s cardiovascular health and risk of disease. Immunology, however, doesn’t have a comparable set of simple measurements that indicate the status of a person’s immune system, Davis says. Some data can provide a rough gauge: Patients with reduced numbers of neutrophils, for instance, are prone to infections. But such data are limited. HIP aims to come up with a uniform group of measurements that can, like a lipid panel, provide a readout of the immune system’s functioning.
A few public and private efforts have scooped up some basic immune data from large numbers of people, including All of Us, the U.S. National Institutes of Health’s program to gather genomic and medical data from 1 million people, and Project Baseline from the Google offshoot Verily, which tallied information on how individuals responded to COVID-19 infection. But such projects have collected limited categories of information and, in Project Baseline’s case, haven’t made the data available publicly.
Another area where research has fallen short is “the understanding of human immune variation and diversity,” says John Tsang, a systems immunologist at Yale University who helped develop HIP’s scientific plan. A litany of factors—including age, sex, diet, living conditions, previous disease exposure, and genetics—shapes how the immune system functions. But most immunological studies are conducted on small, homogenous populations, usually in the United States or Europe, Tsang says. Relying on such a narrow slice of humanity “has biased our understanding,” Thomas says.
HIP aims to address that lack of diversity. “We want baseline data from every human population,” Keirstead says. To capture human variety, HIP’s plans call for up to 300 collection sites on all of the inhabited continents. Each site will measure the same set of variables in as many as 10,000 people, from different socioeconomic levels and a range of ages, from newborns to centenarians. In addition, they will include healthy people as well as individuals who have medical problems such as autoimmune diseases, cancer, and allergies. All volunteers will have to undergo medical exams and provide a detailed health history.
Although HIP intends to begin this global data collection phase in 2027, the effort’s first phase, launching this year, will be smaller and likely involve seven to 10 clinical research centers, including facilities outside the wealthy countries, that are already adept at gathering and analyzing immune data, Keirstead says. At each site, the project will study about 500 people, measuring immune variables including the abundance of different types of immune cells, gene activity, concentrations of metabolic molecules, and DNA sequences. “The idea is that we will go deep and measure as much as possible,” Tsang says. From this mass of data, the project will then select a few variables that provide the clearest picture of how the immune system is working. They will also provide the basis for an immune monitoring kit, a standard set of assays that all the sites in the second part of the project will use.
In the end, HIP will generate nearly 2 trillion immune measurements, which will be publicly available through a central database. With this data haul and other information, HIP will build a predictive AI model that can forecast—based on immune profile, ancestry, economic status, age, and other information—how individuals will respond to stresses or challenges, such as a particular drug or pathogen. The model could help pharmaceutical companies identify opportunities for new treatments and drug reactions to avoid. And by providing a much more detailed view of a population’s health and vulnerability to side effects, the model could enable countries to better decide which drugs are needed by and suitable for their populations, thus allowing them to reduce health care costs, Keirstead says.
What HIP is aiming for with its AI ambitions has “never been done before,” Kierstead says, which is probably why this part of the project draws more skepticism from outside researchers. The project intends to generate not just predictive models, but also ones that replicate how the immune system operates. Mathematical biologist Reinhard Laubenbacher of the University of Florida says the AI will detect patterns of responses but doubts it will open a deeper understanding of the immune system. “Data collection efforts like this are tremendously helpful, but we will probably need more than that,” he says. A priority is “building theoretical framework” to understand the information the project will accrue, he says.
Another challenge is money. To realize its ambitions, HIP will require a supersize budget, about $1 billion to $3 billion over the next 10 years, Keirstead says. To raise the needed funds, HIP now hopes to go beyond its current partners to philanthropies, governments, and other pharmaceutical companies. “I am targeting everyone. There is not going to be a stone left unturned,” he says.
Ensuring that HIP’s far-flung sites follow the same procedures in collecting and analyzing the data will also be a challenge, Lee says, adding that the immune monitoring kits will be a big help in this regard. Thomas says that attracting nonwhite participants could also be difficult, given their mistrust of scientific research like this. “They haven’t seen benefits and have been exploited.”
Still, he and others are eager to see what HIP produces. “If they pull this off, it will be big,” Greenplate says.
Mitchell Leslie of Albuquerque, N.M., is a frequent contributor to Stanford and the journal Science.
Science Magazine.
Support nonprofit science journalism
Help Science publish trustworthy, high-impact stories about research and the people who shape it. Subscribe to News from Science.

The Most Mysterious Cells in Our Bodies Don’t Belong to Us
By Katherine J. Wu
You carry literal pieces of your mom—and maybe your grandma, and your siblings, and your aunts and uncles.
The Atlantic
January 3, 2024

Spread the word